During electrolysis of H2SO4 (aq) with high charge density, H2S2O8 is
Sulfuric acid ( American spelling and the preferred IUPAC name) or sulphuric acid ( Commonwealth spelling ), known in antiquity as oil of vitriol, is a mineral acid composed of the elements sulfur, oxygen, and hydrogen, with the molecular formula H2SO4. It is a colorless, odorless, and viscous liquid that is miscible with water. [6]

Draw the Lewis structure of sulfuric acid H2SO4 with minimized formal
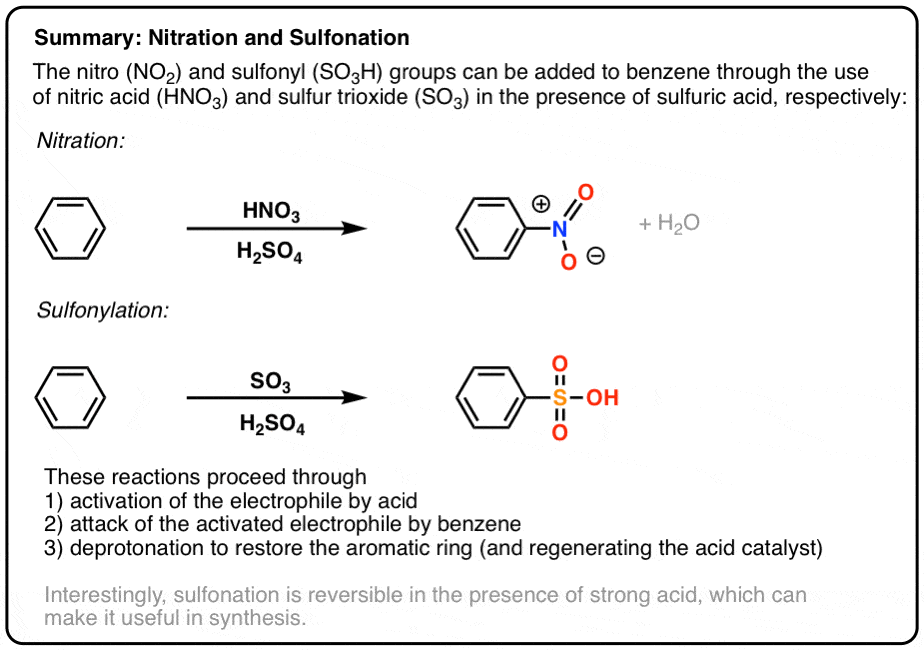
Sulfuric acid is a dibasic strong acid. It means, it can release two hydrogen atoms to show acidic characteristics. Therefore, we can assume, there should be two -OH bonds in sulfuric acid molecule. Lewis structure of H 2 SO 4 Most stable lewis structure of H 2 SO 4 is shown below. Steps of drawing lewis structure of H 2 SO 4

Draw the Lewis structure of H2SO4 Chemistry Chemical Bonding and
How to calculate the formal charges on H2SO4 atoms? The formal charges can be calculated using the formula given below: The formal charge of an atom = [valence electrons of an atom - non-bonding electrons - ½ (bonding electrons)] The valence electrons (V.E) of an atom are the total number of electrons present in its valence shell.

draw the Lewis dot structure of h2so4 Brainly.in
Sulfuric Acid | H2SO4 or H2O4S | CID 1118 - structure, chemical names, physical and chemical properties, classification, patents, literature, biological activities, safety/hazards/toxicity information, supplier lists, and more.
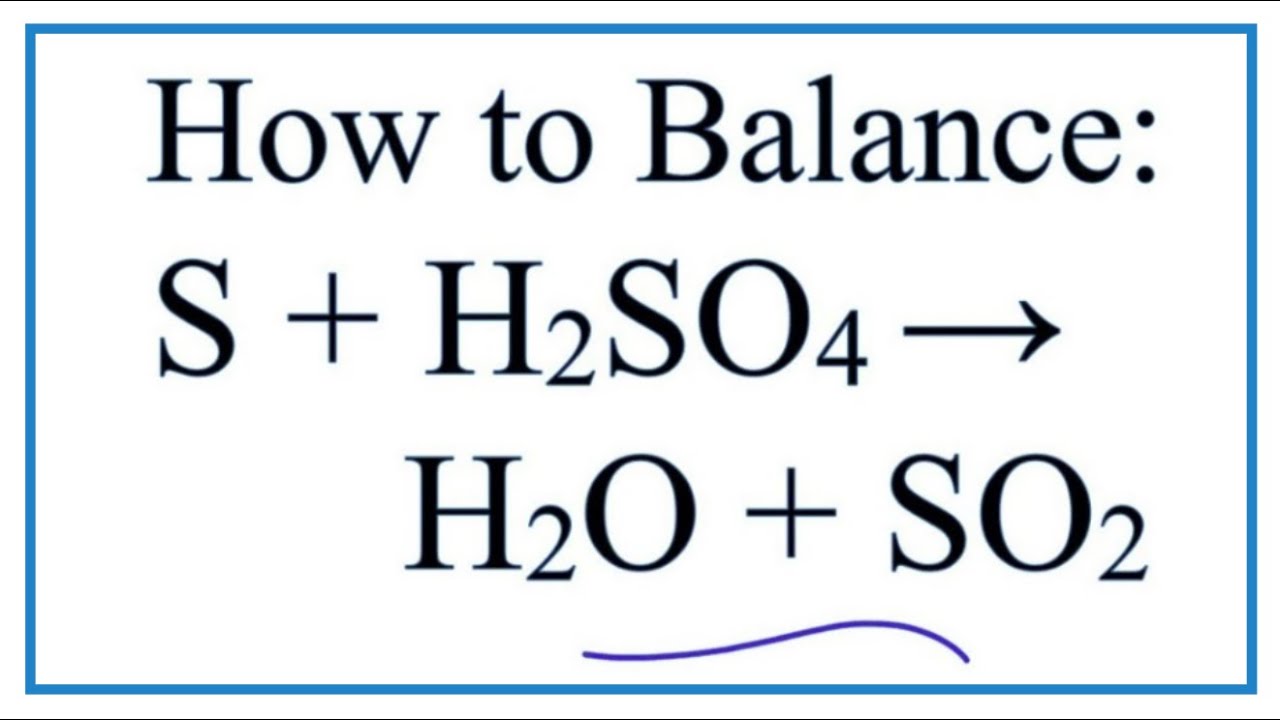
How to Balance S + H2SO4 = H2O + SO2 (Sulfur + Sulfuric acid) YouTube
By Biswarup Chandra Dey This article is regarding the most important acid, H2SO4 lewis structure, and its important facts. Let's start to discuss it. H2SO4 lewis structure is often known as Sulfuric acid. It is known as Oil of Vitriol. In most of the reactions in chemistry, we used sulfuric acid as a reagent. The acidity of H2SO4 is very strong.
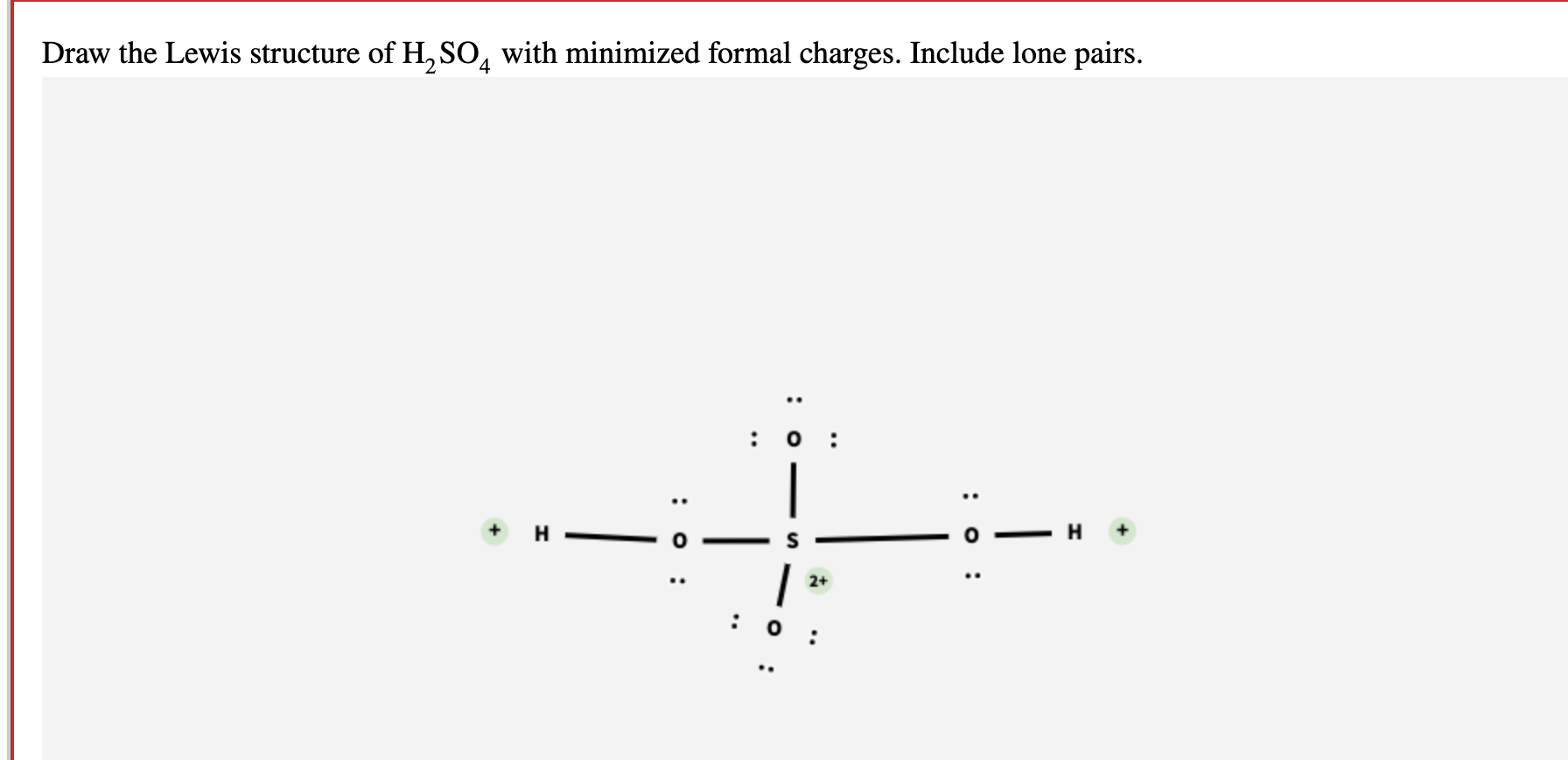
Solved Draw the Lewis structure of H2SO4 with minimized
Structural Formula. H 2 SO 4. sulfuric acid

H2SO4 Lewis Structure Sulfuric Acid YouTube
Chemistry Chemical Compound Formulas Sulfuric Acid Sulfuric Acid - H 2 SO 4 What is Sulfuric Acid? Sulfuric acid (H 2 SO 4) is a strong acid with hygroscopic and oxidizing properties. Sulfuric Acid is a mineral acid with the chemical formula H 2 SO 4. Sulfuric acid is also known as Mattling acid or Oil of vitriol.

How to Write the Net Ionic Equation for H2SO4 + LiOH = Li2SO4 + H2O
And Why? May 26, 2023 by Jay Rana The Charge of H2SO4 (Sulfuric acid) is 0. But the question is how can you say that the charge on H2SO4 (Sulfuric acid) is 0? Well you can say this by calculating its formal charge. So let's calculate the formal charge of H2SO4 (Sulfuric acid).
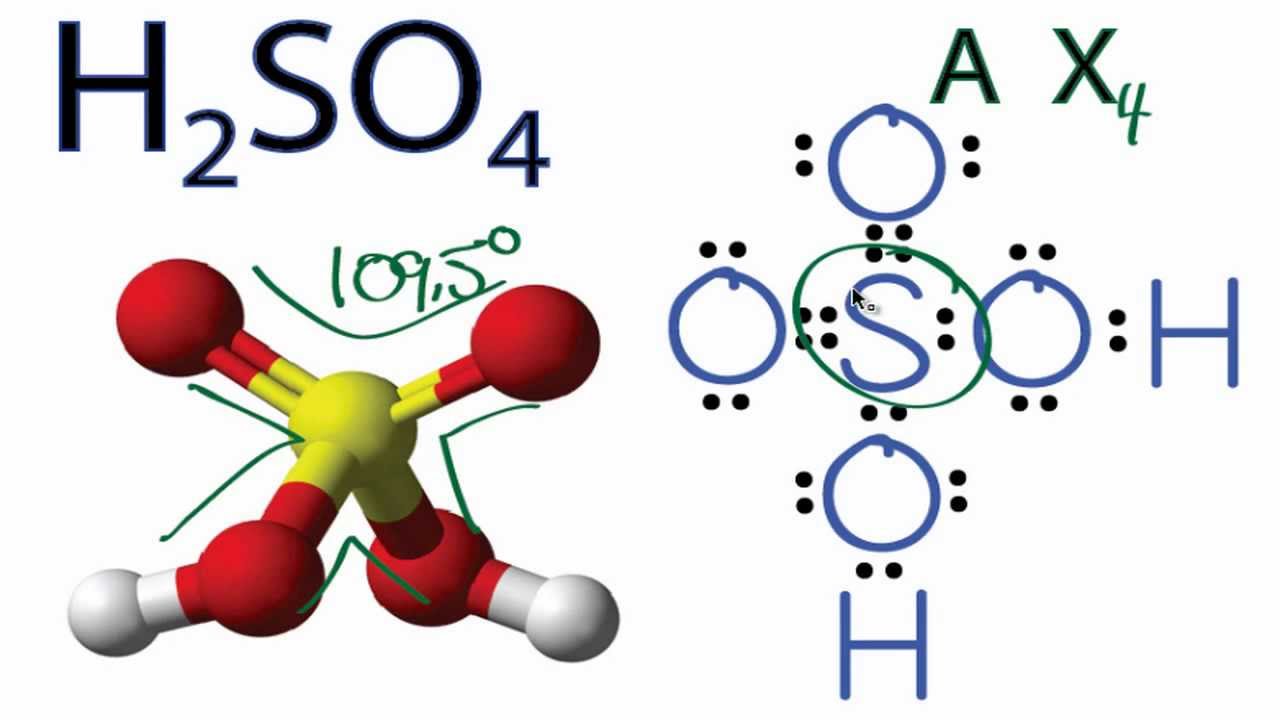
H2SO4 Molecular Geometry / Shape and Bond Angles YouTube
Q. In bisulphate ion, the formal charge on sulphur atom is: Q. What is the formal charge on atom in carbonate and nitric acid? Q. Formal charge on sulphur in SO2 is: Q. In the given, lewis structure of S2O2− 3 formal charge present on sulphur atoms 1 and 2 respectively are: Q. The formal charge on carbon atom in carbonate ion is.
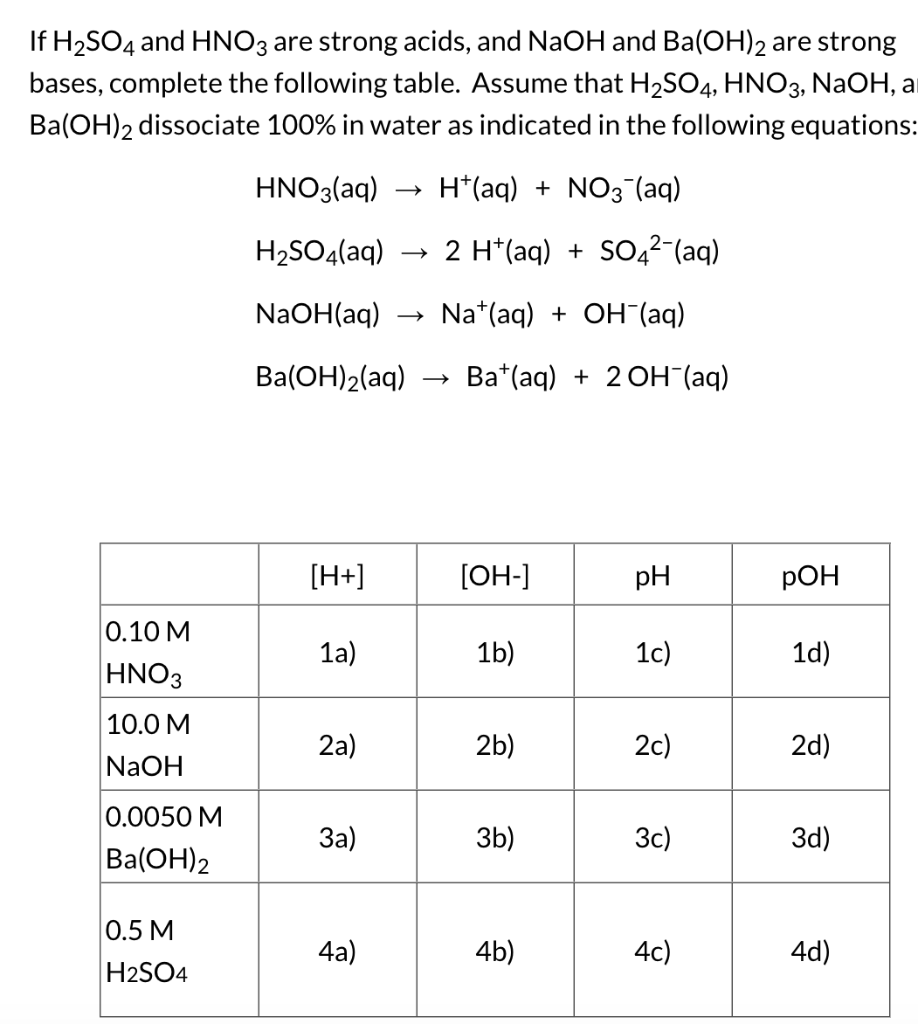
Solved If H2SO4 and HNO3 are strong acids, and NaOH and
In this video we'll write the correct formula for Sulfuric acid.To write the formula for Sulfuric acid we'll use the Periodic Table, a Common Ion Table, and.

Solved Draw The Lewis Structure For Sulfuric Acid, H_2 SO...
It has a molecular weight of 98.079 g/mol. H2SO4 works as an oxidizing and dehydrating agent. Furthermore, it's diprotic in nature which holds the capacity of releasing two protons together. It is colorless, odorless, and extremely corrosive in nature.
H2so4 Conjugate Base Lewis Structure Drawing Easy
In order to calculate the formal charges for H2SO4 we'll use the equation:Formal charge = [# of valence electrons] - [nonbonding val electrons] - [bonding el.

How to Calculate the Formal Charges for H2SO4 (Sulfuric acid) YouTube
The concentration of acetic acid in the final solution will drop below 0.10 M, but the total of the two species must equal 0.10 M, the initial amount that was put into solution. [Acetic acid] + [acetate] = 0.10 M. The charge balance must account for all positively charged (sodium and hydronium ions) and negatively charged (acetate and hydroxide.

H2SO4 là gì? AXIT SUNFURIC HÓA CHẤT CÔNG NGHIỆP QUAN TRỌNG NHẤT HIỆN
Quantity Value Units Method Reference Comment; Δ r H°-132.3 ± 2.9: kJ/mol: RSC: Blanchard, Joly, et al., 1974: solvent: Sulphuric acid aqueous solution; The reaction enthalpy relies on -10.6 kJ/mol for the enthalpy of solution of EtOH(l) and on 35.1±0.1 for the enthalpy of solution of K2SO4(cr) Blanchard, Joly, et al., 1974.; MS
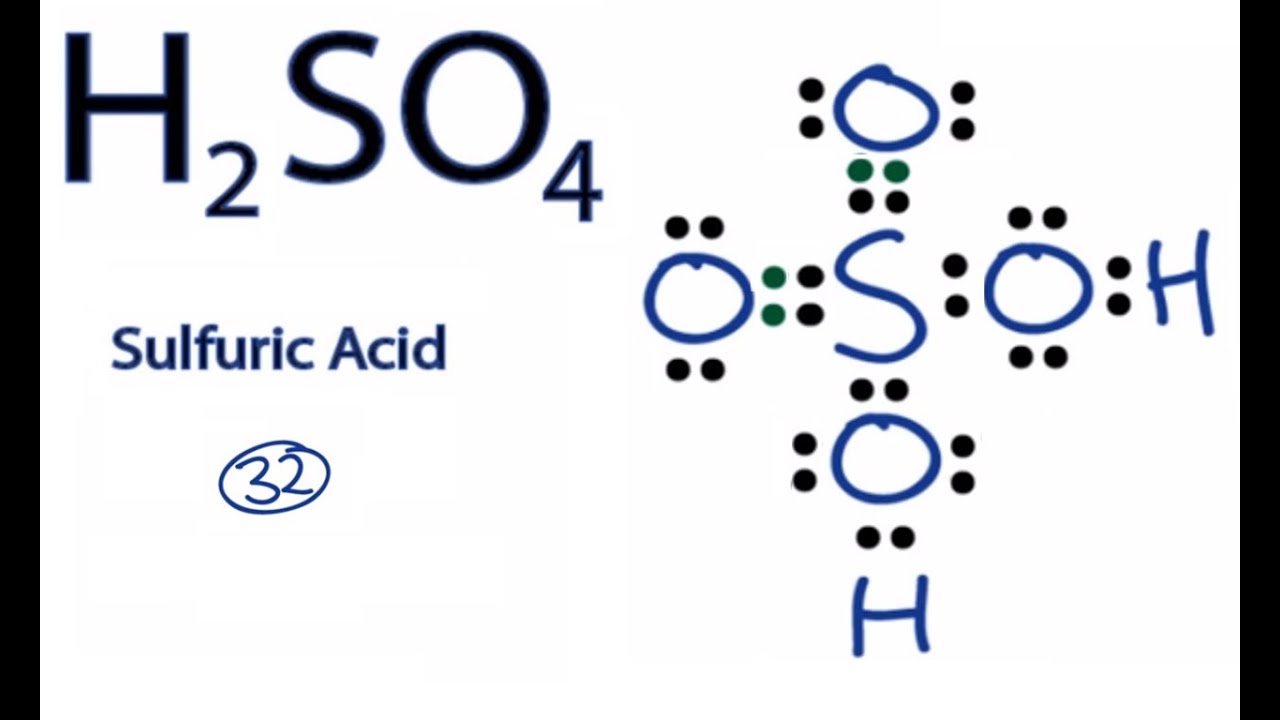
lewis dot structure for h2so4
The acid-base strength of a molecule depends strongly on its structure. The weaker the A-H or B-H+ bond, the more likely it is to dissociate to form an H+ H + ion. In addition, any factor that stabilizes the lone pair on the conjugate base favors the dissociation of H+ H +, making the conjugate acid a stronger acid.
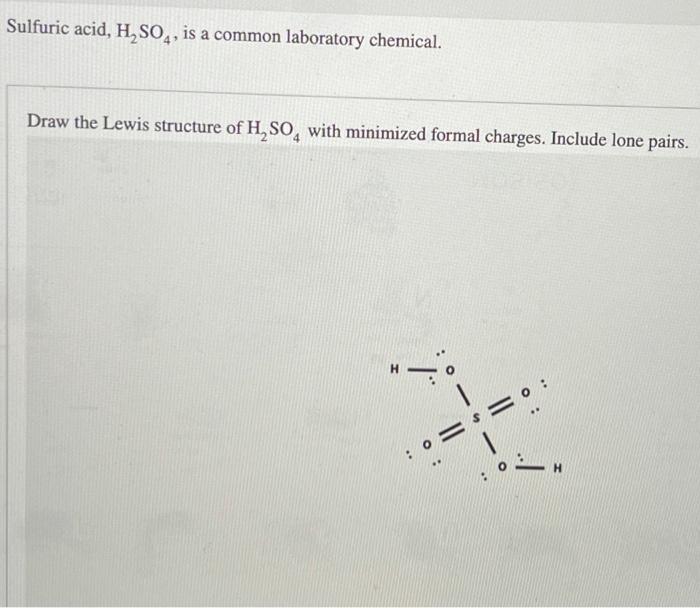
(Get Answer) Sulfuric Acid, H2SO4, Is A Common Laboratory Chemical
In the $\ce{H2SO4}$ molecule, two bonds are simple covalent ($\ce{S-OH}$ ones), and two are dative ($\ce{S-O}$ ones).. $\begingroup$ A structure with four double bonds in the sulphate ion would give the sulphur atom a formal charge of -2. This is not expected to be more stable than a configuration with two double bonds and two single bonds.