
What Is Ch3cl Lewis Structure?
Check me out: http://www.chemistnate.com

Chloromethane Molecule Stock Illustrations 42 Chloromethane Molecule
In the Lewis structure, each hydrogen has a zero placed nearby while the nitrogen has a +1 placed nearby. Adding together the formal charges on the atoms should give us the total charge on the molecule or ion. In this case, the sum of the formal charges is 0 + 1 + 0 + 0 + 0 = +1. Exercise 9.5.2 9.5. 2.

Chloromethane Methyl Chloride Stock Vector Illustration of chloride
Chloromethane (CH3Cl) is a stable compound where the atoms are in a stable condition and do not easily react with other elements under normal conditions. The Lewis structure can easily help with predicting the molecular geometry, hybridization, polarity, and a molecular orbital diagram for the CH3Cl molecule.
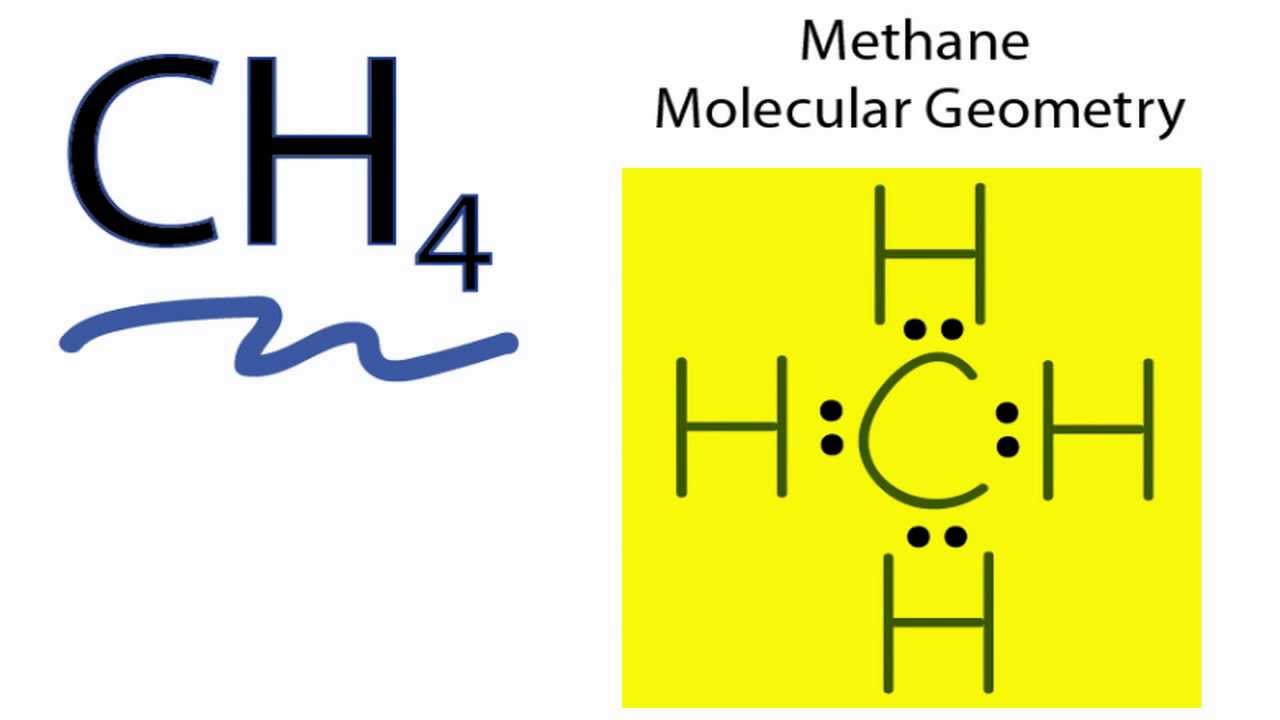
Lewis Structure Ch4 Polar Or Nonpolar Key Polar and onpolar
In OF 2, each atom has an octet as drawn, so nothing changes. Example 10.4.1 10.4. 1: Writing Lewis Structures. NASA's Cassini-Huygens mission detected a large cloud of toxic hydrogen cyanide (HCN) on Titan, one of Saturn's moons. Titan also contains ethane (H 3 CCH 3 ), acetylene (HCCH), and ammonia (NH 3 ).
Chloromethane Molecule Photograph by Molekuul/science Photo Library
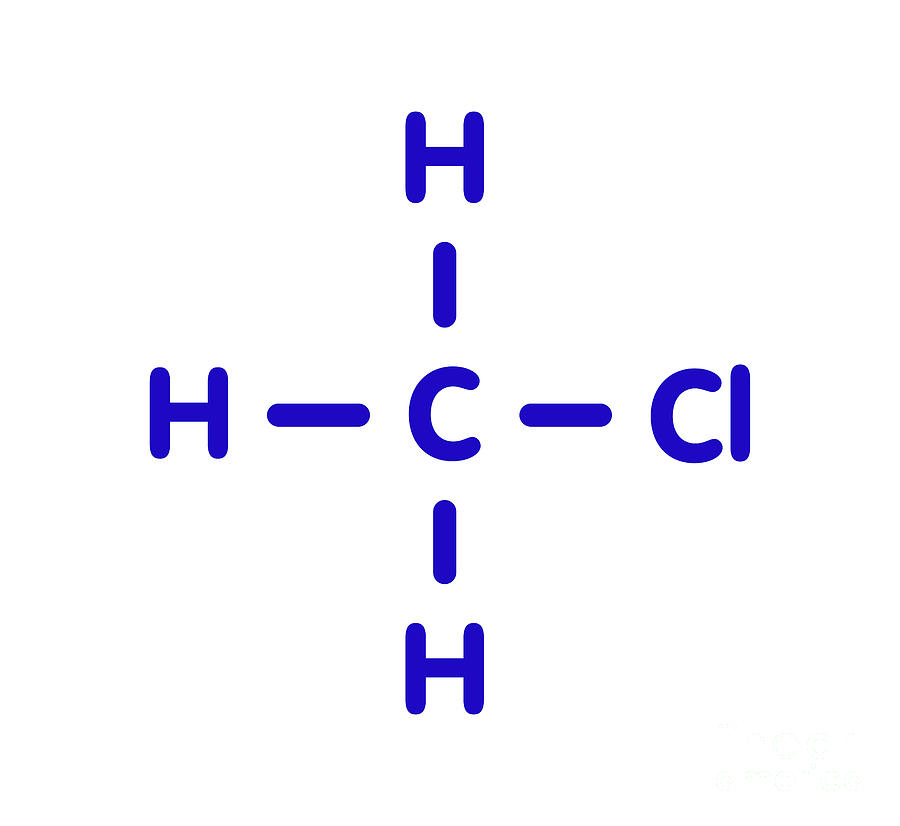
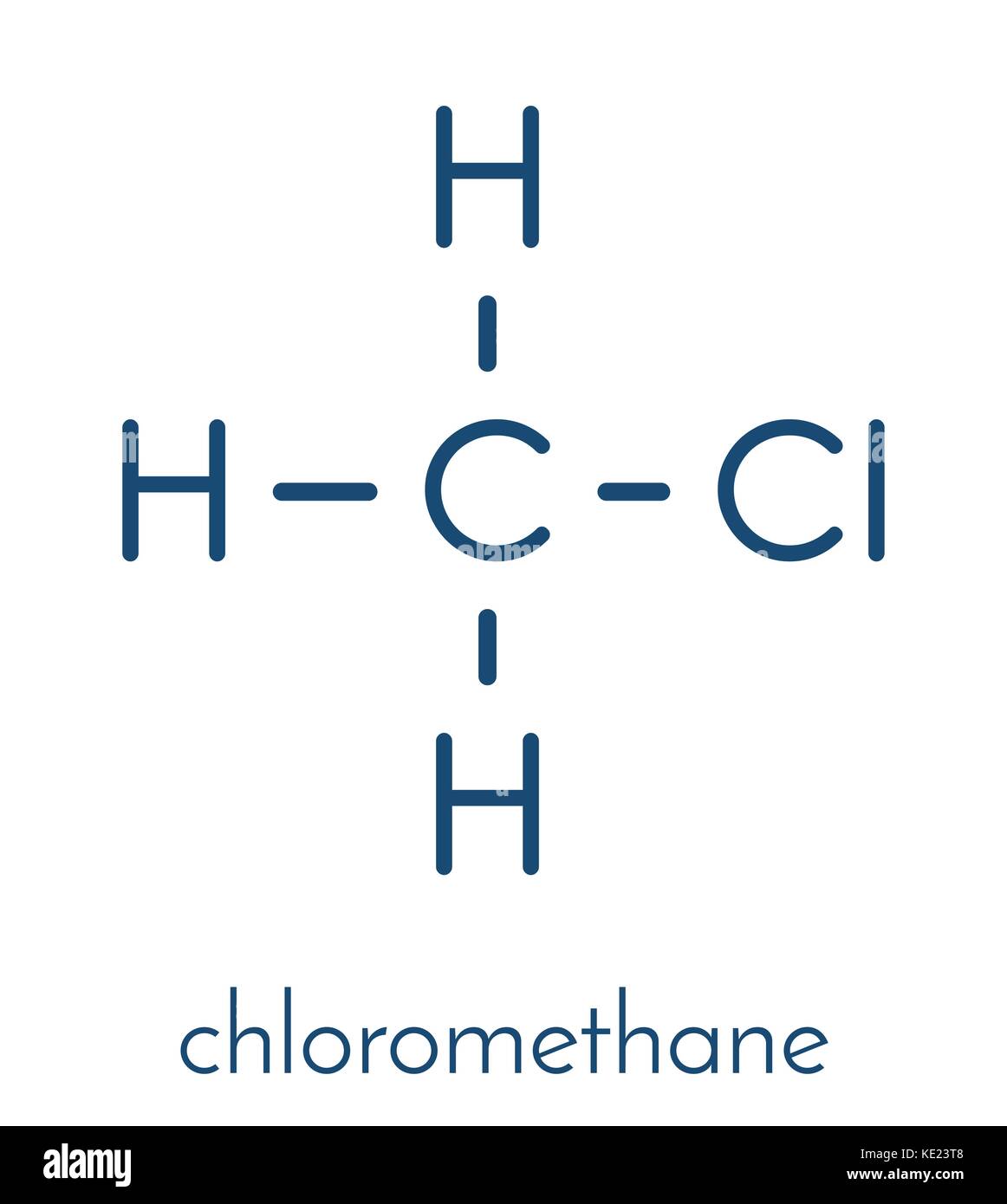
The Lewis structure of XeF 4 indicates six regions of high electron density around the xenon atom: two lone pairs and four bonds: These six regions adopt an octahedral arrangement (Figure 7.19), which is the electron-pair geometry.. Chloromethane, CH 3 Cl, is a tetrahedral molecule with three slightly polar C-H bonds and a more polar C-Cl.

[Solved] Draw a diagram to show the shape of 9to5Science
CHCl3 Hybridization. The concept of hybridization explains the geometrical shape and bonding in polyatomic molecules. An orbital is a 3D region around the nucleus where the probability of finding an electron is maximum. Hybridization can be defined as the mixing of pure atomic orbitals to form hybrid atomic orbitals.

electron dot structure of chloromethane Brainly.in
Methyl chloride has been identified as a chemical component of tobacco smoke (1). The methyl chloride concentration in tobacco smoke collected in canisters was about 30-500 ppmv (1.5-5.3 mg/cigarette) compared with about 500 parts per trillion volume in typical urban air (2).

So far, we’ve used 14 of the CH3Cl Lewis structure’s total 14 outermost
The molecule is symmetric. The two oxygen atoms pull on the electrons by exactly the same amount. Propane is nonpolar, because it is symmetric, with H atoms bonded to every side around the central atoms and no unshared pairs of electrons. Exercise 4.12. 1. Label each of the following as polar or nonpolar.

CH3Cl Lewis Structure (Chloromethane) Chemistry 10, Molecules, Lewis
Chloromethane. Molecular Formula CHCl. Average mass 50.487 Da. Monoisotopic mass 49.992329 Da. ChemSpider ID 6087.

Draw The Structure Of C H C H Electronic Dot Structure Chemistry My
In the Lewis structure of CH3Cl, Carbon is at the central position and all the other atoms around it. The bond angles of Carbon with Hydrogen and Chlorine atoms are 109.5 degrees. This molecule has a tetrahedral shape, and the central carbon atom has sp3 hybridization.
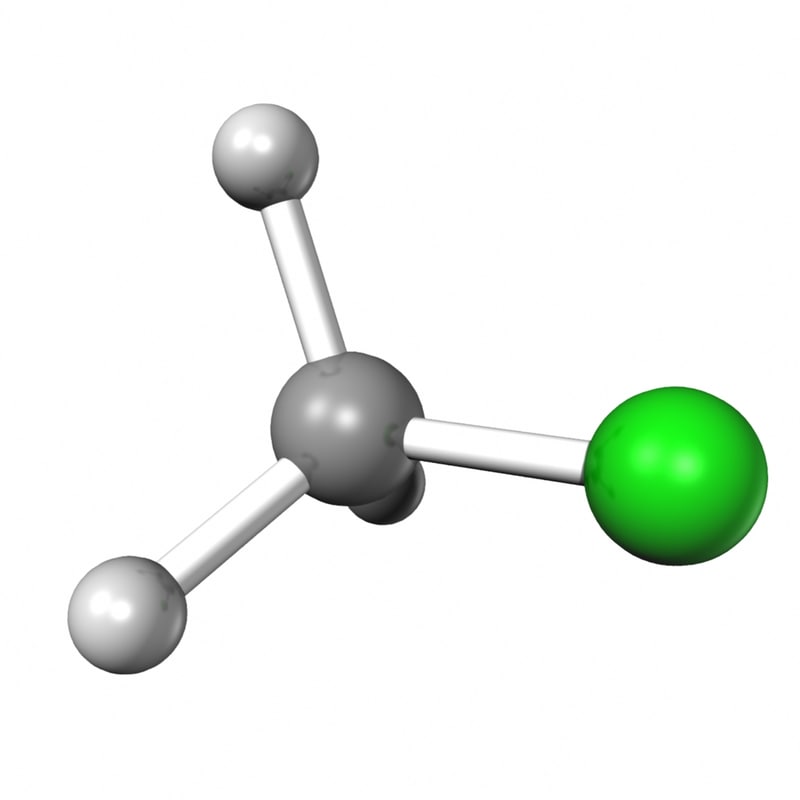
ch3cl 3d obj
An explanation of the molecular geometry for the CH3Cl (Chloromethane or Methyl chloride) including a description of the CH3Cl bond angles. The electron geom.
Chloromethane (methyl chloride) molecule. Skeletal formula Stock Vector
Chloromethane. Formula: CH 3 Cl. Molecular weight: 50.488. IUPAC Standard InChI: IUPAC Standard InChIKey:NEHMKBQYUWJMIP-UHFFFAOYSA-N. CAS Registry Number: 74-87-3. Chemical structure: This structure is also available as a 2d Mol file or as a computed 3d SD file The 3d structure may be viewed using Java or Javascript .
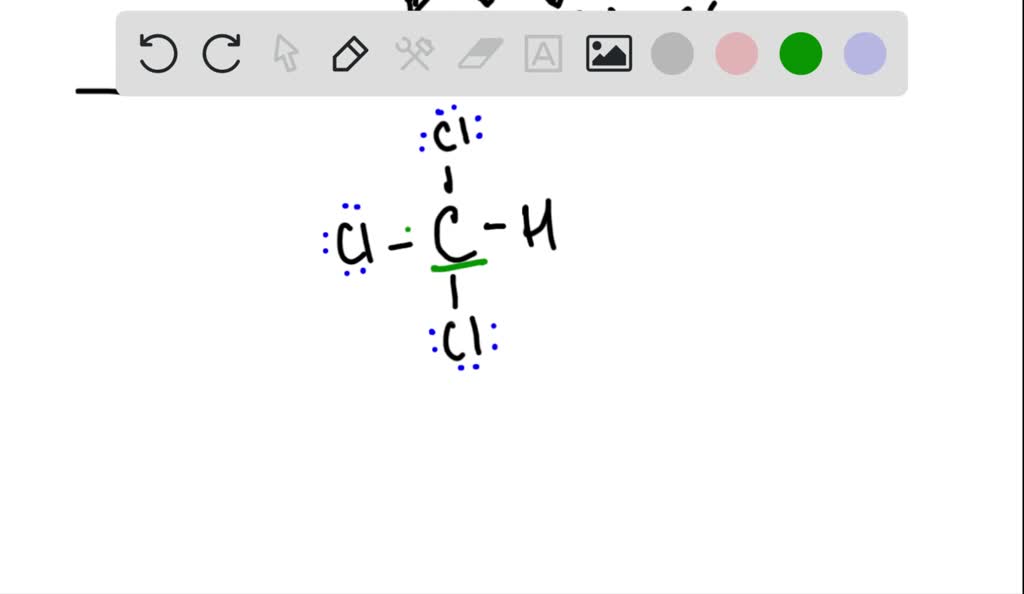
Draw the Lewis structure for chloroform, CHCl_. What … SolvedLib
A chemical structure of a molecule includes the arrangement of atoms and the chemical bonds that hold the atoms together. The Chloromethane molecule contains a total of 4 bond (s). There are 1 non-H bond (s). Images of the chemical structure of Chloromethane are given below: 2-dimensional (2D) chemical structure image of Chloromethane.

Difference Between Chloroform and Dichloromethane Compare the
The information on this page is fact-checked. CH 3 Cl (chloromethane) has one carbon atom, three hydrogen atoms, and one chlorine atom. In the CH 3 Cl Lewis structure, there are four single bonds around the carbon atom, with three hydrogen atoms and one chlorine atom attached to it, and on the chlorine atom, there are three lone pairs.

Chloromethane Image & Photo (Free Trial) Bigstock
3. Cl) Lewis Structure. Chloromethane (CH 3 Cl) contains one carbon atom, three hydrogen atoms and one chlorine atom. In the lewis structure of CH 3 Cl, carbon atom is located as the center atom and other atoms have made bonds with carbon atom.

Chloromethane molecule Stock Image F012/0884 Science Photo Library
A step-by-step explanation of how to draw the CH3Cl Lewis Dot Structure (Chloromethane).For the CH3Cl structure use the periodic table to find the total numb.