Uses of Metals and Non Metals [in Daily life] Teachoo Concepts
In general, the number of valence electrons is the same within a column and increases from left to right within a row. Group 1 elements have just one valence electron and group 18 elements have eight, except for helium, which has only two electrons total. Thus, group number is a good predictor of how reactive each element will be:
Helium atom diagram concept Royalty Free Vector Image
The exception is helium, which has two valence electrons. This periodic table shows the valences of element groups. The transition metals make use of the d-subshell, which can accommodate 10 electrons. The f-subshell holds 14 electrons and the g-subshell contains up to 18 electrons. Metals in the middle of the periodic table become more stable.
How To Find the Helium Electron Configuration (He)
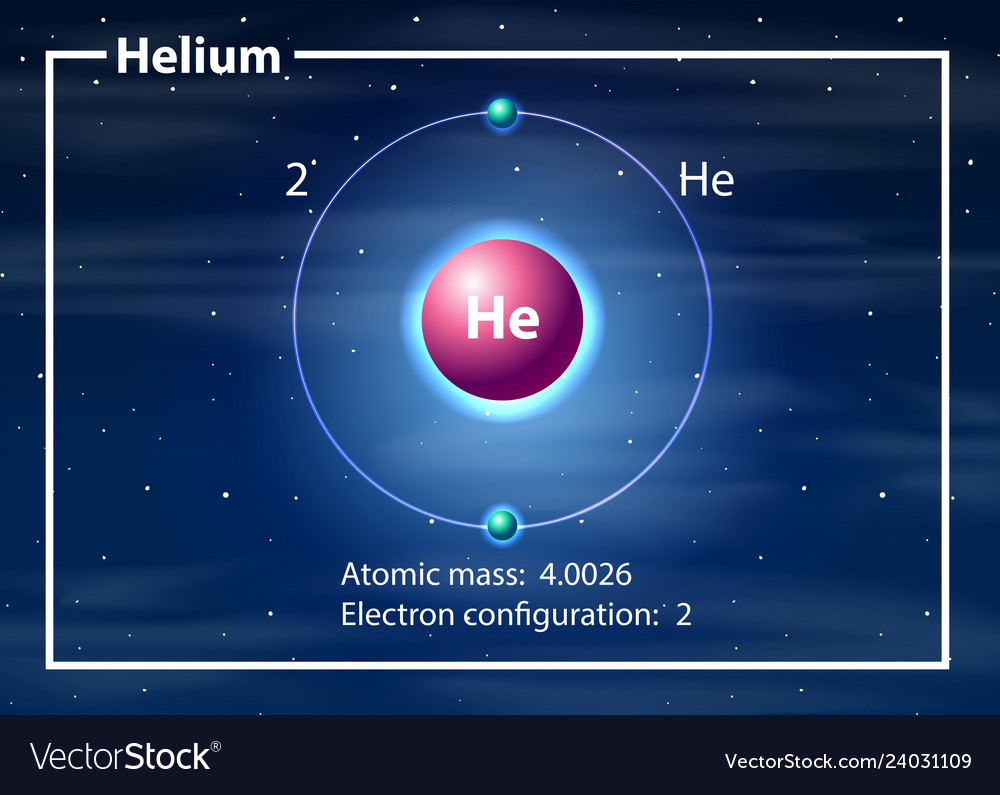
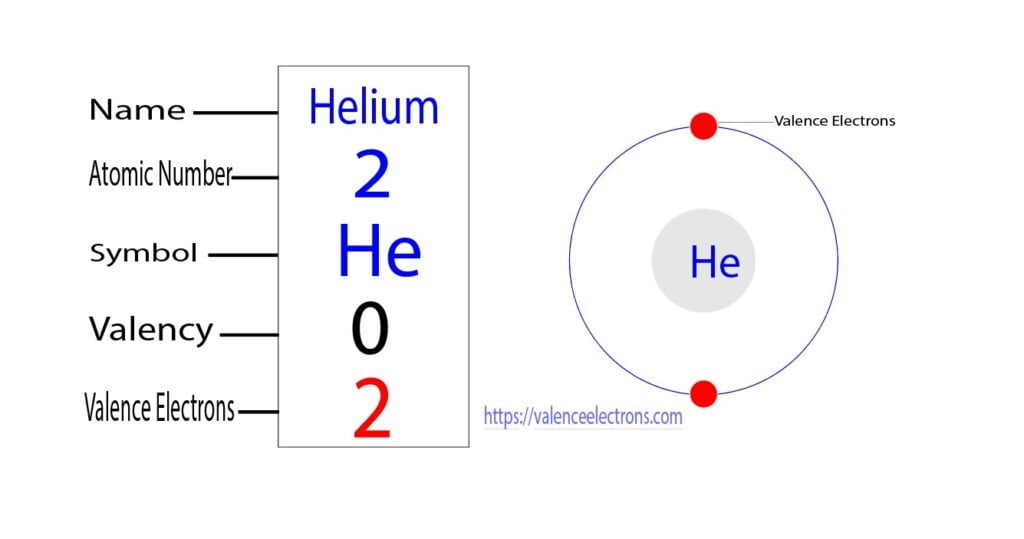
Calcium would have two valence electrons, since it is in Group IIA (Group 2). Helium is the only exception for the main group elements. The first energy level holds a maximum of two valence electrons. Since helium atoms only have two electrons and the outermost energy level is the first energy level, there can only be two valence electrons.

How to Find the Valence Electrons for Helium (He) YouTube
Valence bond theory describes a chemical bond as the overlap of atomic orbitals. In the case of the hydrogen molecule, the 1s orbital of one hydrogen atom overlaps with the 1s orbital of the second hydrogen atom to form a molecular orbital called a sigma bond which contains two electrons of opposite spin. The mutual attraction between this.
Electron Shell Helium Atom Valence Electron Electron Configuration PNG
Bohr diagrams indicate how many electrons fill each principal shell. Group 18 elements (helium, neon, and argon are shown in Figure 2) have a full outer, or valence, shell. A full valence shell is the most stable electron configuration. Elements in other groups have partially filled valence shells and gain or lose electrons to achieve a stable.

Protons, Neutrons, Electrons for Helium Complete Guide
About Transcript Valence electrons are the electrons in the outermost shell, or energy level, of an atom. For example, oxygen has six valence electrons, two in the 2s subshell and four in the 2p subshell. We can write the configuration of oxygen's valence electrons as 2s²2p⁴. Created by Sal Khan. Questions Tips & Thanks
Elements and Atoms The Building Blocks of Matter · Anatomy and Physiology
AboutTranscript. The s-, p-, and d-block elements of the periodic table are arranged into 18 numbered columns, or groups. The elements in each group have the same number of valence electrons. As a result, elements in the same group often display similar properties and reactivity. Created by Sal Khan.
Electron of the Element Helium Stock Vector Illustration of electron
The electron dot diagram for helium, with two valence electrons, is as follows: \[\mathbf{He}\mathbf{:} \nonumber \] By putting the two electrons together on the same side, we emphasize the fact that these two electrons are both in the 1s subshell; this is the common convention we will adopt, although there will be exceptions later.
How To Find The Valence Electrons For Helium (He)?
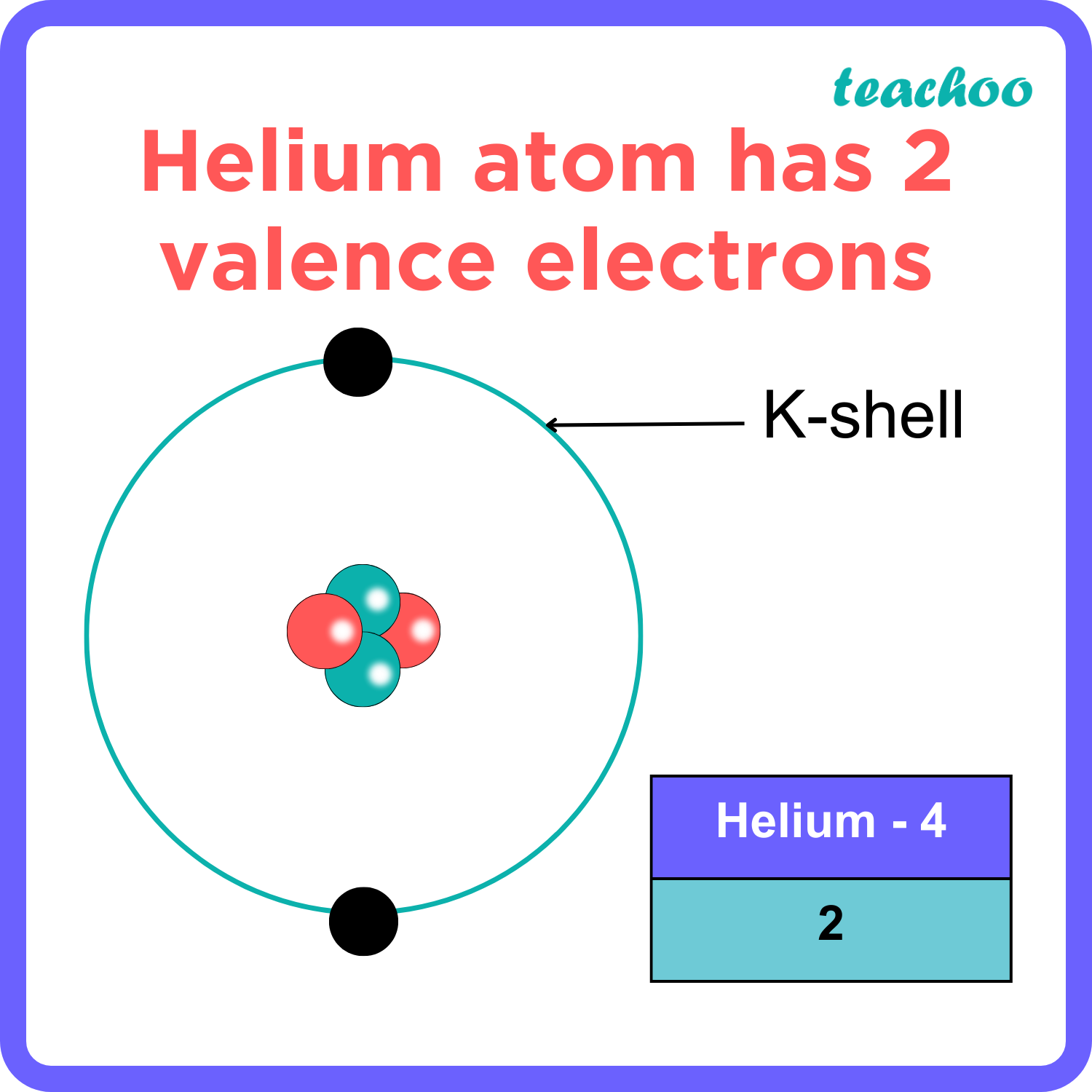
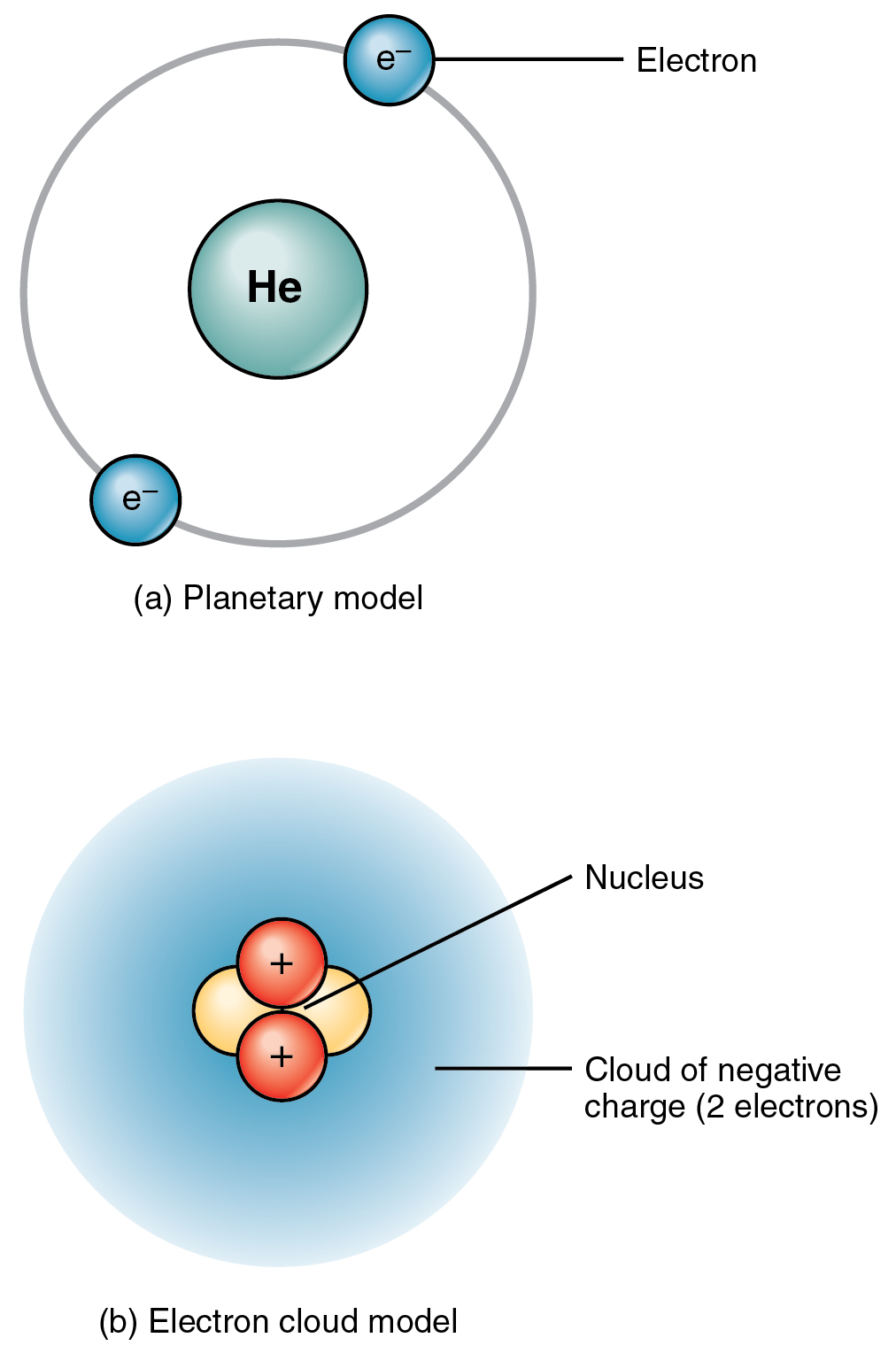
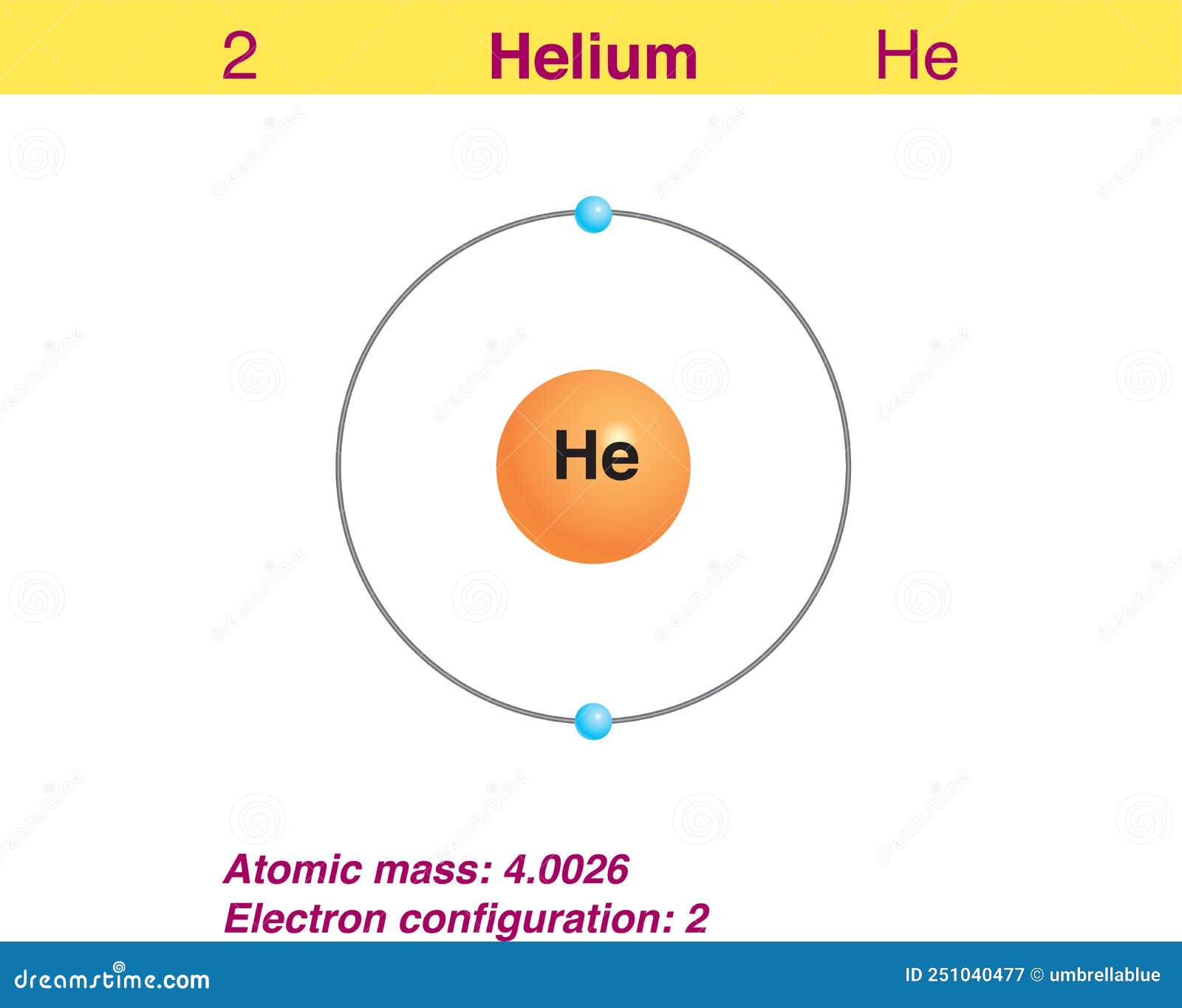
The total number of electrons in the last shell of helium is called the valence electron of helium. The total number of electrons in the last shell of an element after electron configuration is called the valence electrons. The valence electrons are in the last shell of the element. Helium (He) atom (Bohr model)
Protons, Neutrons, Electrons for Helium Complete Guide
Explanation: Helium (He) has the highest ionization energy because, like other noble gases, helium's valence shell is full. Therefore, helium is stable and does not readily lose or gain electrons. 2. Answer: A.) True Explanation: Atomic radius increases from right to left on the periodic table. Therefore, nitrogen is larger than oxygen. 3.
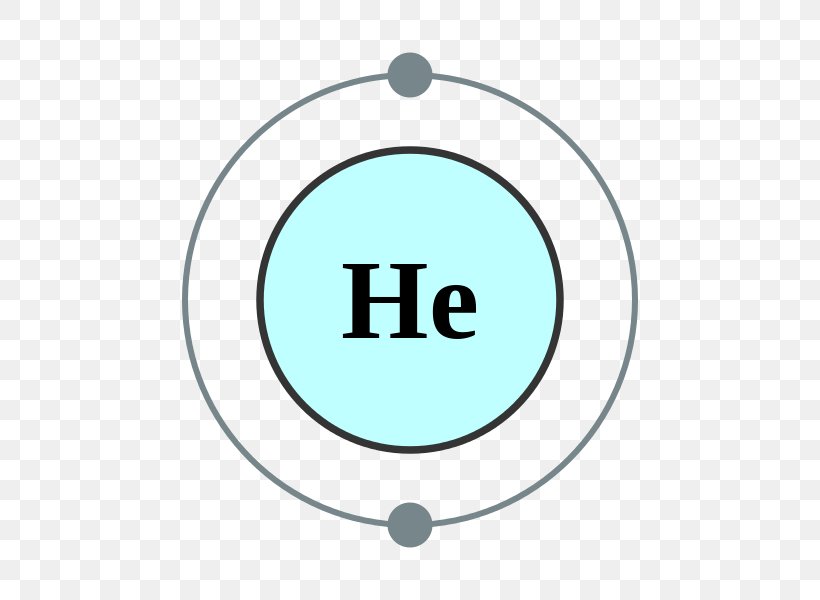
Electron Shell Helium Atom Valence Electron Electron Configuration, PNG
There are two ways to find the number of valence electrons in Helium (He). The first is to use the Periodic Table to figure out how many electrons Helium has.
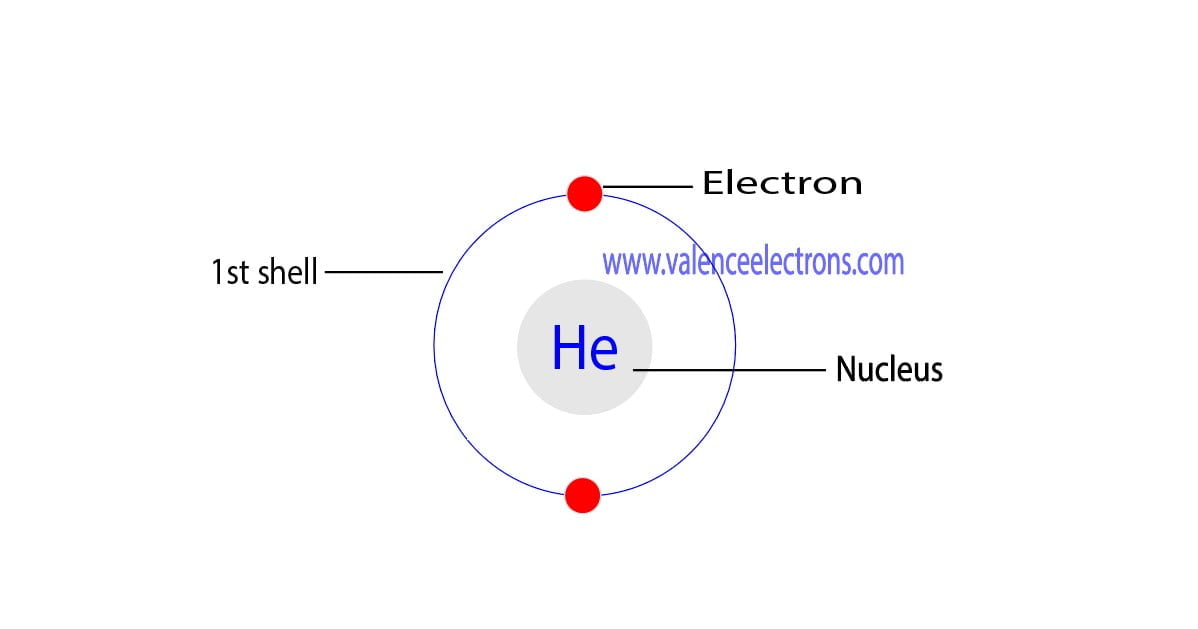
How To Find The Valence Electrons For Helium (He)?
If helium is in group 8 why does it not have 8 valence electrons . And when two hydrogen atoms combine they make a molecule and then have a total of 2 valence electrons if they are then stable then why are the elements in group 2 not stable with 2 valence electrons ? • Comment ( 13 votes) Upvote Downvote Flag Just Keith 9 years ago

Helium Valence Electrons In Helium
Helium is composed of two electrons in atomic orbitals surrounding a nucleus containing two protons and (usually) two neutrons. As in Newtonian mechanics,. Helium II has no such valence band but nevertheless conducts heat well.
How Many Valence Electrons Does Helium (He) Have? [Valency of He]
You can see in the electron configuration of helium ( 1s2) that the highest energy level is 1. And the total number of electrons present in this energy level is 2. So by knowing the electron configuration, we have found that the Helium has 2 valence electrons. I hope you have understood the methods of finding the valence electrons in helium.

Helium, atomic structure Stock Image C018/3683 Science Photo Library
How many valence electrons does helium have? Chemistry The Periodic Table Valence Electrons 2 Answers Stefan V. Feb 17, 2015 2. Explanation: Helium is located in period 1, group 18 of the Periodic Table and has an atomic number equal to 2. As a result, neutral helium will only have 2 electrons surrounding its nucleus.
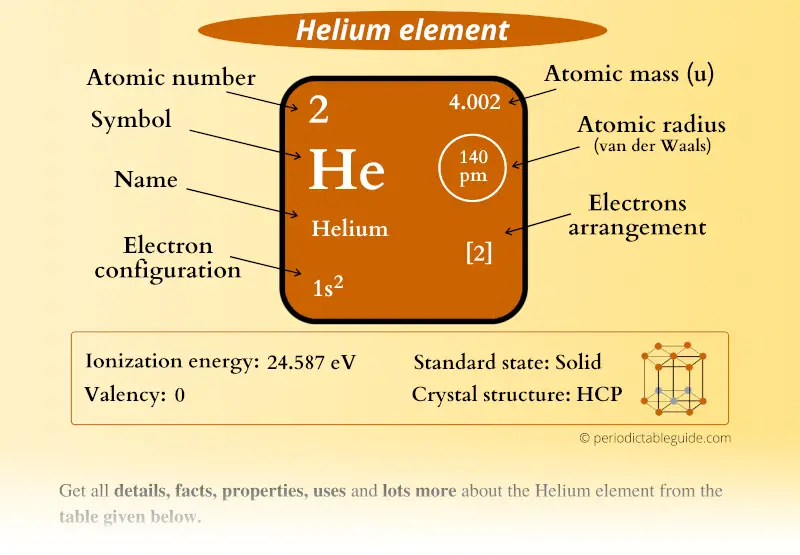
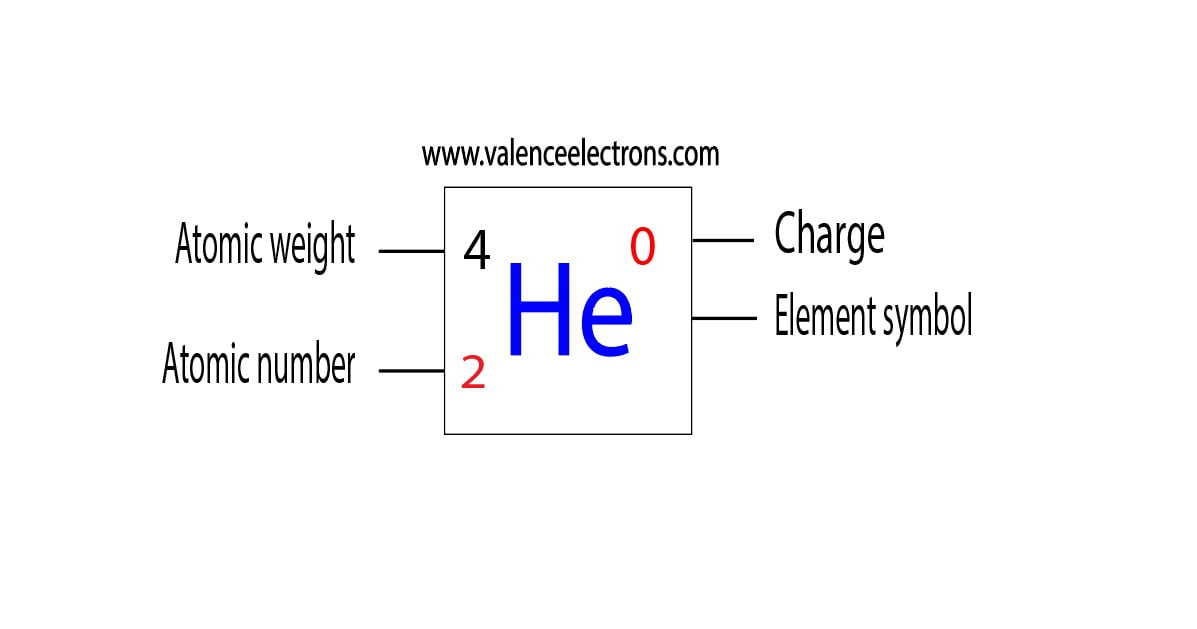
Helium Element in Periodic table (Info + Why not in group 2)
An atom's valence electrons are the electrons in its outermost shell. In the chlorine model below, the valence electrons are shown in red . The number of valence electrons determines most of an atom's chemical behaviors. So, it's important to be able to identify how many valence electrons atoms of different elements have.