
Sodium Carbonate (Soda Ash) Jinlei Chemical
Molecular Formula Na2CO3 CNa2O3 Synonyms SODIUM CARBONATE 497-19-8 Soda Ash Disodium carbonate Carbonic acid disodium salt View More. Molecular Weight 105.988 g/mol Computed by PubChem 2.2 (PubChem release 2021.10.14) Parent Compound CID 767 (Carbonic Acid) Component Compounds CID 5360545 (Sodium) CID 767 (Carbonic Acid) Dates Create: 2005-07-19
PPT Analysis of Soda Ash PowerPoint Presentation, free download ID
Sodium carbonate, also known as soda ash or washing soda, is a chemical compound with the formula Na2CO3. It is a white, crystalline solid that is commonly used in various industries. Sodium carbonate has a wide range of applications, including as a cleaning agent, pH regulator, and water softener.It is also used in the production of glass, paper, and textiles.
The Chemistry of LimeSoda Ash Precipitation Water Softening HubPages
Soda Ash (Sodium carbonate), Na 2 CO 3 .10H 2 O, is an industrial chemical with the formula Na2CO3. It is also known as washing soda, soda ash, and soda crystals. All of the forms are water-soluble, colorless, odorless salts that produce moderately alkaline solutions in water. Soda Ash What is Soda Ash (Sodium Carbonate) used for?
Sodium Carbonate, Na2CO3, Natrium Carbonate, Washing Soda, Soda Ash
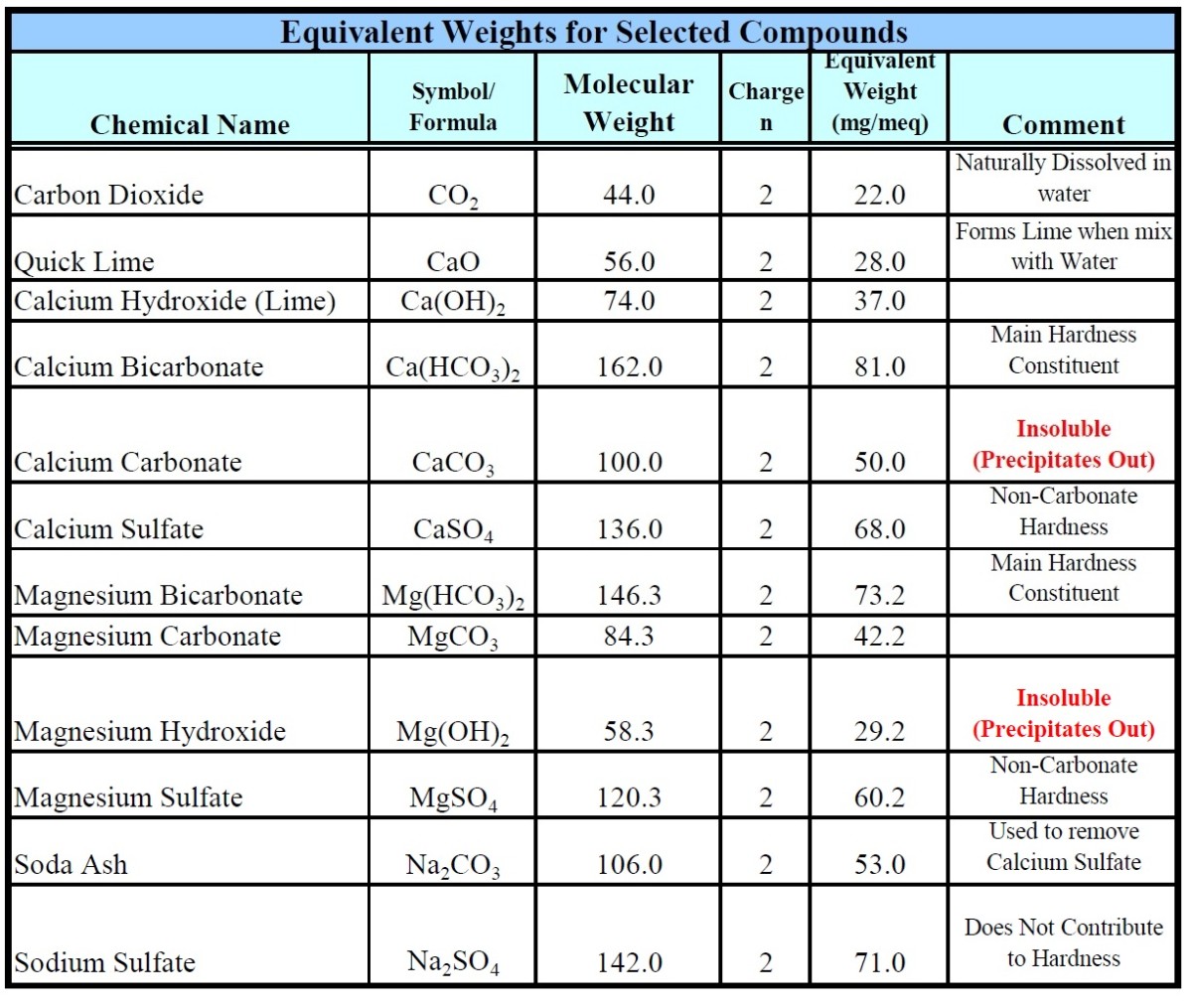
The chemical formula of soda ash is Na2CO3. The molar mass of anhydrous soda ash is roughly equal to 106 grams per mole. Under standard conditions for temperature and pressure (often abbreviated to STP), soda ash is known to exist as a white solid that is hygroscopic in nature. Is baking soda same as soda ash?

Soda Ash 10lbs Tie Dye Sodium Carbonate Washing Soda Stain
Sodium carbonate, commonly known as soda ash in its anhydrous form, is the sodium salt of carbonic acid with the chemical formula Na 2 CO 3 . It occurs naturally in arid regions and is often extracted from mineral deposits of trona ore. Wyoming is the largest domestic source, with large natural trona deposits being mined.

Soda Ash Complete Information Including Health Benefits, Selection
It is carbonic acid's sodium salt with the chemical formula Na2CO3 N a 2 C O 3. It is frequently extracted from mineral resources of trona ore, where it naturally exists. The greatest domestic source, with sizable natural trona resources being extracted, is Wyoming.

Soda Ash
Simply heat baking soda or sodium bicarbonate in a 200 F oven for about an hour. Carbon dioxide and water will be given off, leaving dry sodium carbonate. This is the soda ash. The chemical reaction for the process is: 2 NaHCO 3 (s) → Na 2 CO 3 (s) + CO 2 (g) + H 2 O (g)
PPT Analysis of Soda Ash PowerPoint Presentation, free download ID
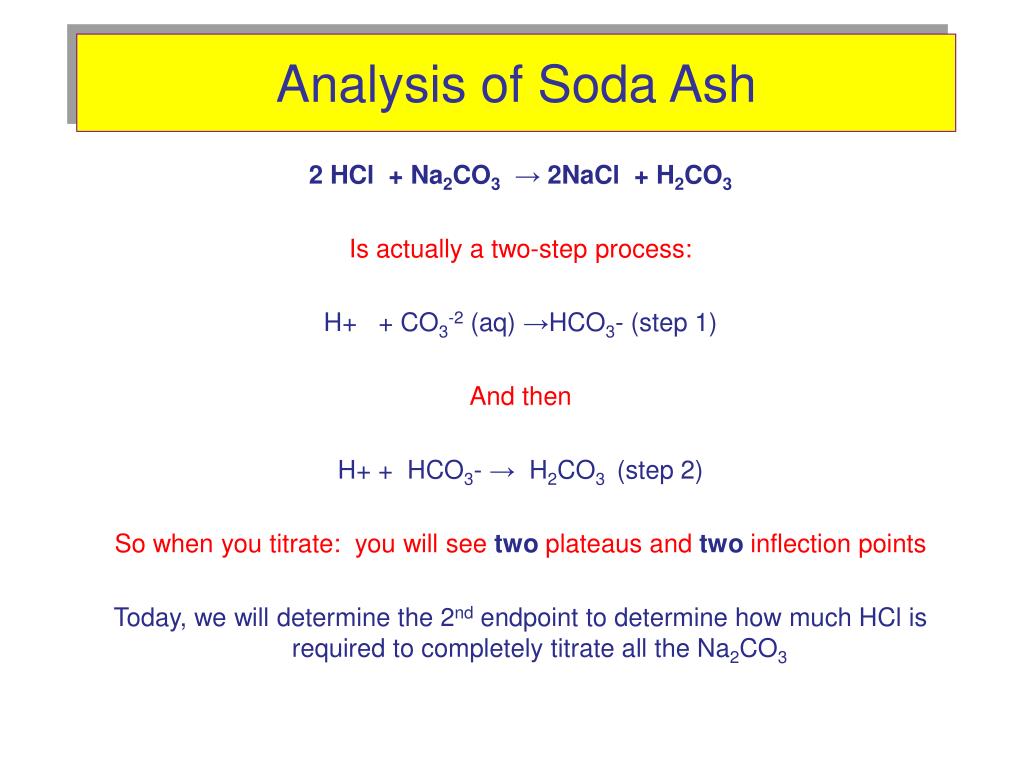
The titration involved in the determination of the carbonate content is an example of a weak base being titrated with a strong acid. The two weak bases involved are C O 3 2 − with K b 1 = 2.1 × 10 4 and H C O 3 − with K b 2 = 2.4 × 10 − 8. The reactions involved are: (1) C O 3 2 − + H 3 O + ⇌ H C O 3 − + H 2 O. and.
Sodium carbonate, Na2CO3, natrium carbonate, washing soda, soda ash
Let's have a look at some key differences between sodium carbonate and sodium bicarbonate. Sodium carbonate is often referred to as soda ash or washing soda. Sodium bicarbonate is popularly called as baking soda. . . Sodium carbonate is made up of sodium and acid. Sodium bicarbonate comes with sodium, acid and hydrogen.

Washing soda Acids, bases, and salts Chemistry Khan Academy YouTube
The chemical formula of soda ash is Na 2 CO 3. The molar mass of anhydrous soda ash is roughly equal to 106 grams per mole. The decahydrate of this compound is known to have a molar mass of 286.14 grams per mole.

Bulk Soda Ash Chemical Formula Na2co3 Buy Soda Ash,Soda Ash Chemical
Sodium carbonate (also known as washing soda, soda ash and soda crystals) is the inorganic compound with the formula Na2CO3 and its various hydrates. All forms are white, odourless, water-soluble salts that yield alkaline solutions in water.

Sodium Carbonate / Washing Soda / Soda Ash Chemical Formula China
Solvay process The Solvay process or ammonia-soda process is the major industrial process for the production of sodium carbonate (soda ash, Na 2 CO 3 ). The ammonia-soda process was developed into its modern form by the Belgian chemist Ernest Solvay during the 1860s. [1]

Soda Ash The Second Largest End Use of Salt
Soda Ash is the 10th most consumed inorganic compound in the world, which has been used for over 5,000 years. It is a safe, simple compound and a key component in a variety of industrial processes from the manufacture of glass to dry powder detergents and lithium-ion batteries. It is also an important ingredient in the food and pharmaceutical.

Soda Ash.docx Sodium Carbonate Chemical Substances Free 30day
ABOUT SODA ASH. Soda ash, the trade name for sodium carbonate (Na2CO3), is a white, anhydrous, powdered or granular material. It is an essential raw material used in the manufacture of glass, detergents and soaps, chemicals and other industrial products. As such, soda ash is one of the most widely used and important commodities in the United.

What Is the Difference between Baking Soda and Soda Ash? Uses & More
Soda ash The preparation of Sodium Carbonate is also known as Solvay process. But before that calcium carbonate (limestone) was heated to release carbon dioxide. Thirdly, anhydrous sodium carbonate or soda ash is dissolved in water and recrystallized to get washing soda crystals containing 10 molecules of water of crystallization. Na2CO3.

Soda ash chemical formula is
Sodium carbonate. Sodium carbonate (also known as washing soda or soda ash ), Na 2 CO 3, is a sodium salt of carbonic acid. It most commonly occurs as a crystalline heptahydrate, which readily effloresces to form a white powder, the monohydrate. It has a cooling alkaline taste, and can be extracted from the ashes of many plants.