paleontology Montessori Muddle
Atomic Structure of Oxygen. Properties of Oxygen. Physical Properties of Oxygen. Oxygen, denoted by the chemical symbol O, is a fundamental element known for its life-sustaining properties. Here are the detailed physical properties of oxygen: Phase: Oxygen is a gas at standard temperature and pressure (STP). However, it can be converted into a.
Forms of Energy ND Studies Energy Level 2
Occurrence and properties. At 46 percent of the mass, oxygen is the most plentiful element in Earth's crust. The proportion of oxygen by volume in the atmosphere is 21 percent and by weight in seawater is 89 percent. In rocks, it is combined with metals and nonmetals in the form of oxides that are acidic (such as those of sulfur, carbon, aluminum, and phosphorus) or basic (such as those of.

Diagram representation of the element oxygen Vector Image
Figure 2.2.1 2.2. 1: The Structure of the Atom. Atoms have protons and neutrons in the center, making the nucleus, while the electrons orbit the nucleus. The modern atomic theory states that atoms of one element are the same, while atoms of different elements are different.

Oxygen atomic structure, artwork Stock Image C016/4341 Science Photo Library
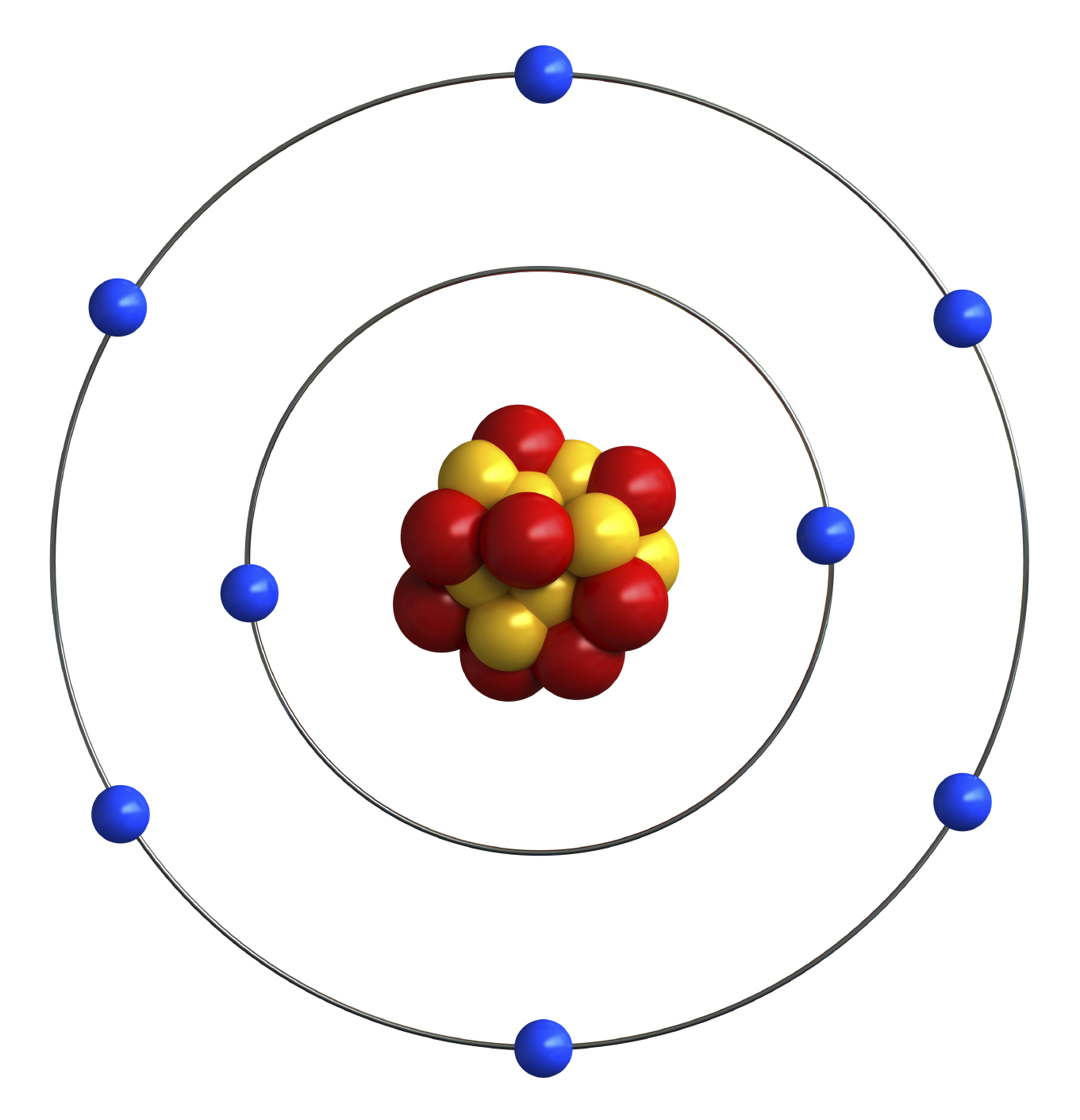
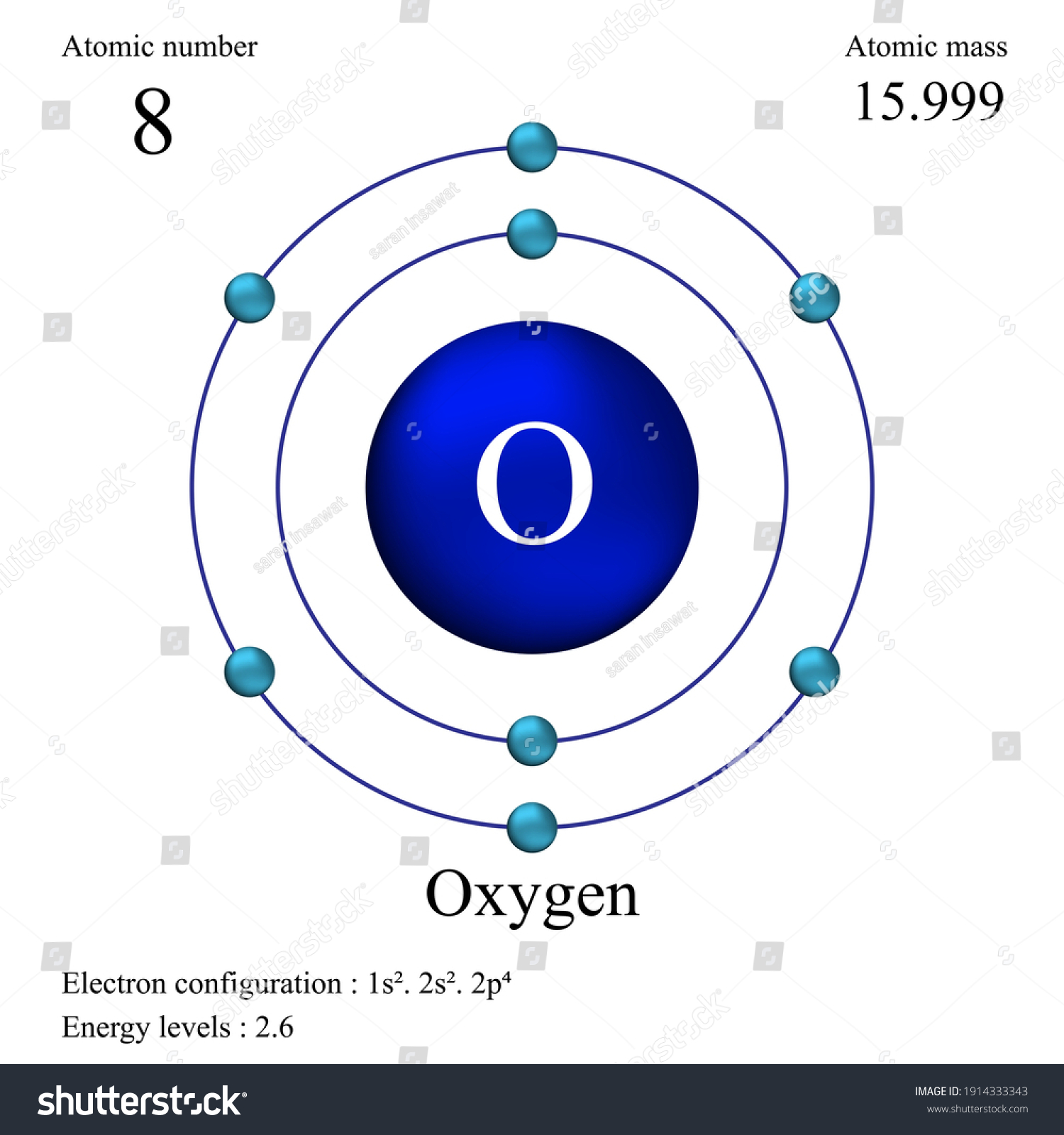
Atomic Number of Oxygen. Oxygen is a chemical element with atomic number 8 which means there are 8 protons and 8 electrons in the atomic structure. The chemical symbol for Oxygen is O. The atom consist of a small but massive nucleus surrounded by a cloud of rapidly moving electrons. The nucleus is composed of protons and neutrons.

29 Diagram Of Oxygen Atom Wiring Diagram Info
Oxygen is a chemical element. It has the symbol O and atomic number 8. It is the third most common element in the universe, after hydrogen and helium. Oxygen is more than a fifth of the Earth's atmosphere by volume. In the air, two oxygen atoms usually join to make dioxygen (O 2), a colourless gas. This gas is often just called oxygen.

Oxygen atomic structure, artwork Stock Image C016/4342 Science Photo Library
Atomic structure - AQA Structure of the atom. Atoms consist of a nucleus containing protons and neutrons, surrounded by electrons in shells. The numbers of subatomic particles in an atom can be.
Atomic Model Of Oxygen ClipArt Best
Oxygen is a chemical element; it has symbol O and atomic number 8. It is a member of the chalcogen group in the periodic table, a highly reactive nonmetal, and an oxidizing agent that readily forms oxides with most elements as well as with other compounds. Oxygen is the most abundant element in Earth's crust, and after hydrogen and helium, it is the third-most abundant element in the universe.

Oxygen, atomic structure Stock Image C013/1508 Science Photo Library
Oxygen, atomic. Molecular Formula O. Average mass 15.999 Da. Monoisotopic mass 15.994915 Da. ChemSpider ID 140526.
Bohr Model Chemical Element Oxygen Atomic Theory, PNG, 1200x1200px, Bohr Model, Area, Atom
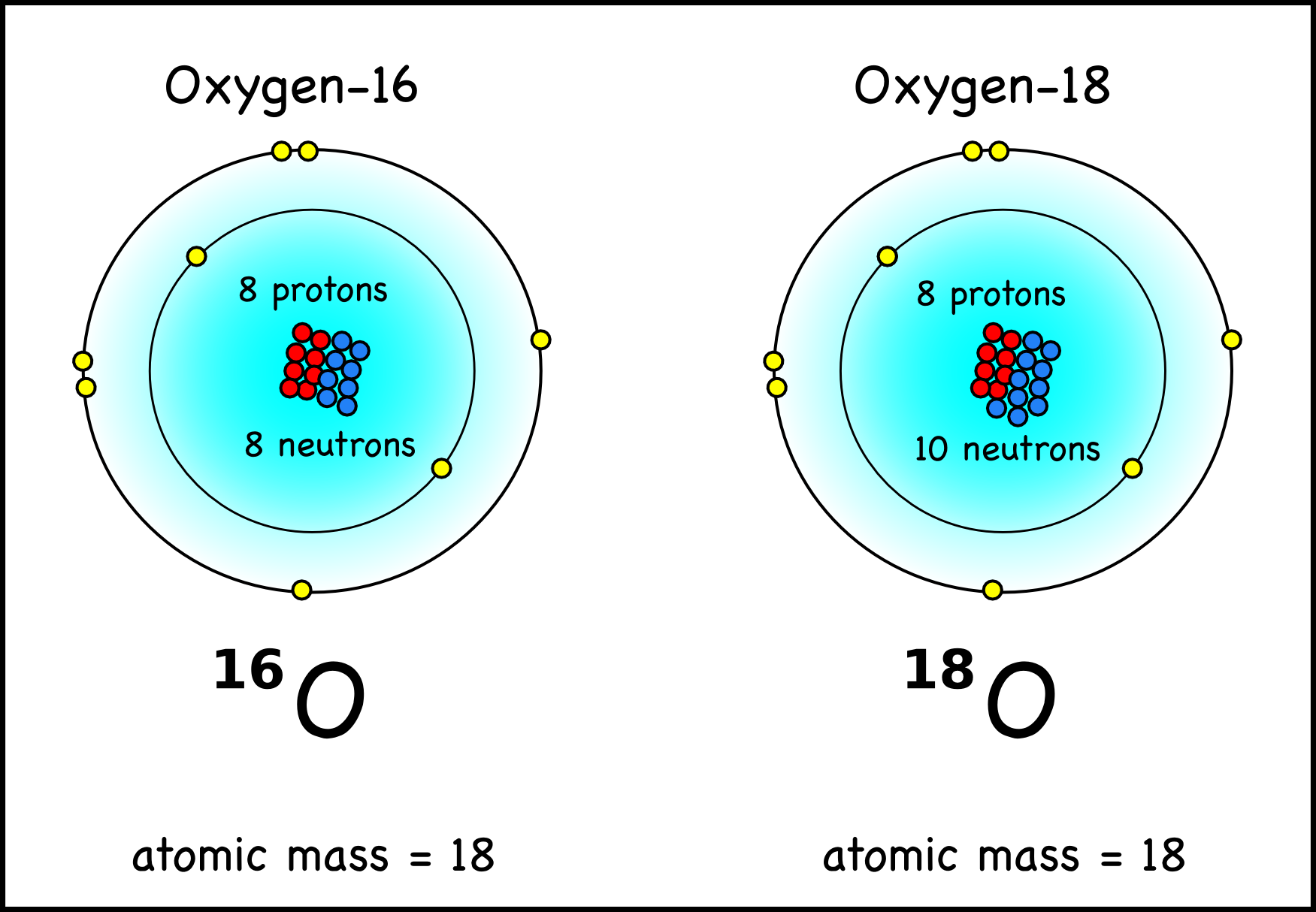
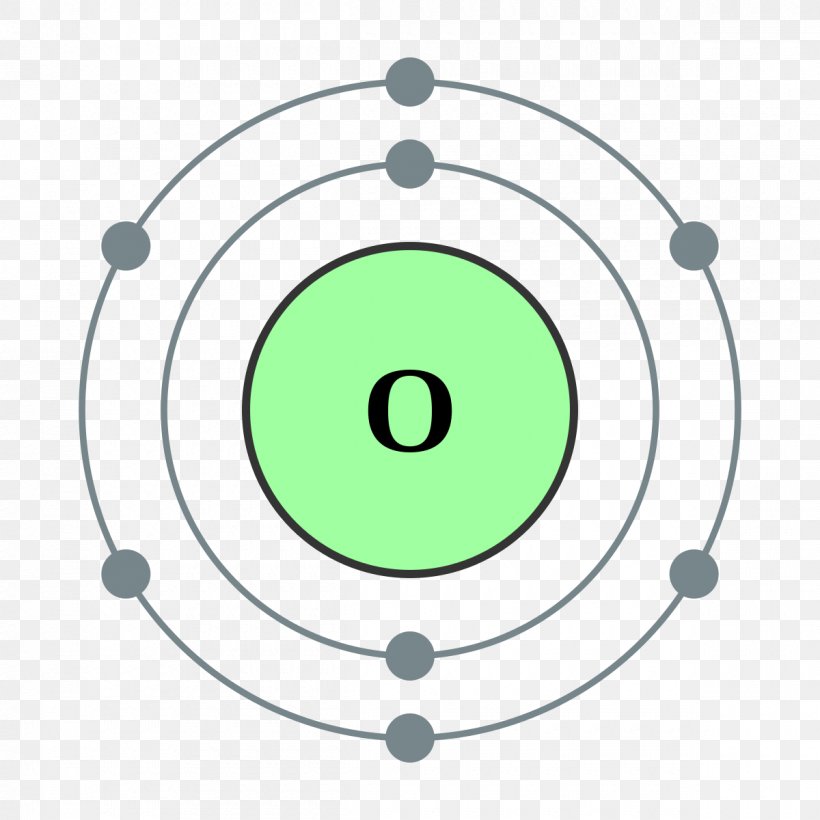
An Oxygen atom has: 8 protons. 8 electrons. 8 neutrons. To know more about the atomic structure of oxygen, you need to learn about the electronic configuration. The electronic configuration shows the distribution of electrons in an atom. And, it can be shown in two ways: In the form of shells. In the form of orbitals.

FileElectron shell 008 Oxygen.svg Wikimedia Commons Atom diagram, Electron configuration
Oxygen is a chemical element - a substance that contains only one type of atom. Its official chemical symbol is O, and its atomic number is 8, which means that an oxygen atom has eight protons in its nucleus. Oxygen is a gas at room temperature and has no colour, smell or taste. Oxygen is found naturally as a molecule. Two oxygen atoms strongly bind together with a covalent double bond to.
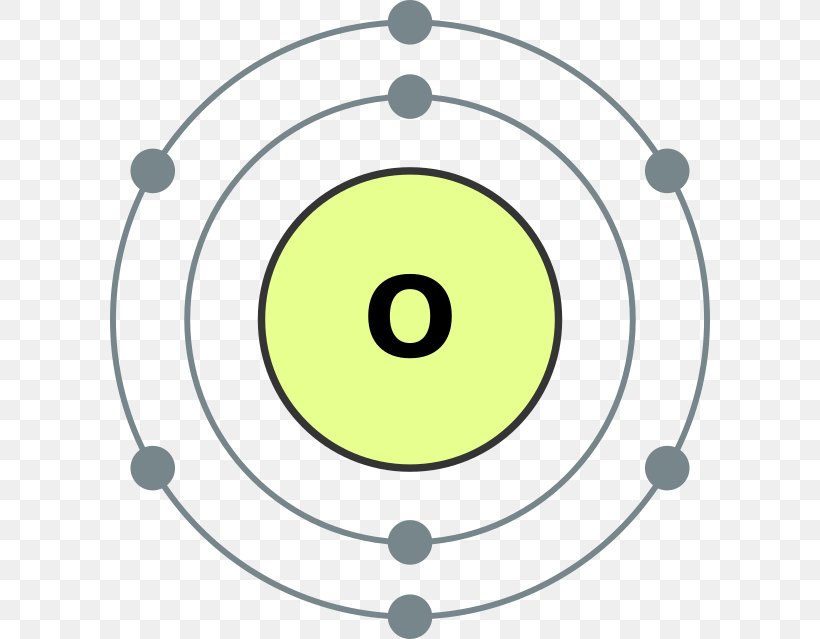
Bohr Model Atomic Number Oxygen Diagram, PNG, 600x639px, Bohr Model, Area, Atom, Atomic Number
Know everything about Oxygen Facts, Physical Properties, Chemical Properties, Electronic configuration, Atomic and Crystal Structure. Oxygen is a chemical element with symbol O and atomic number 8. It is a member of the chalcogen group on the periodic table and is a highly reactive nonmetal and oxidizing agent that readily forms compounds (notably oxides) with most elements.
Atom diagram Images, Stock Photos & Vectors Shutterstock
Magnetic Properties of Oxygen. Oxygen (O 2) is paramagnetic.An oxygen molecule has six valence electrons, so the O 2 molecule has 12 valence electrons with the electron configuration shown below:. As shown, there are two unpaired electrons, which causes O 2 to be paramagnetic. There are also eight valence electrons in the bonding orbitals and four in antibonding orbitals, which makes the bond.

Why does oxygen form the "O"_2^(2) ion? Socratic
Atomic structure . Atomic structure signifies the structure of an atom encompassing a nucleus in which the protons are positively charged and neutrons that are neutral are present.The negatively charged particles are called electrons which spin around the center of the nucleus.; The addition of the total number of protons and neutrons existing in the nucleus of an atom is known as the mass.

Oxygen, atomic structure Stock Image C045/6426 Science Photo Library
In this video we'll look at the atomic structure and Bohr model for the Oxygen atom (O). We'll use a Bohr diagram to visually represent where the electrons a.

oxygen atom Chuba Oyolu's Portfolio
Oxygen is a chemical element; it has symbol O and atomic number 8. It is a member of the chalcogen group in the periodic table, a highly reactive nonmetal, and an oxidizing agent that readily forms oxides with most elements as well as with other compounds. Oxygen is the most abundant element in Earth's crust, and after hydrogen and helium, it.

Oxygen Atom Science Notes and Projects
Oxygen is an element displayed by the symbol O, and atomic number 8. It is an essential element for human survival. Decreased oxygen levels may be treated with medical oxygen therapy. Treatment with oxygen serves to increase blood oxygen levels and also exerts a secondary effect of decreasing blood flow resistance in the diseased lung, leading to decreased cardiovascular workload in an attempt.