
So far, we’ve used 8 of the Cl2 Lewis structure’s total 14 outermost valence shell electrons
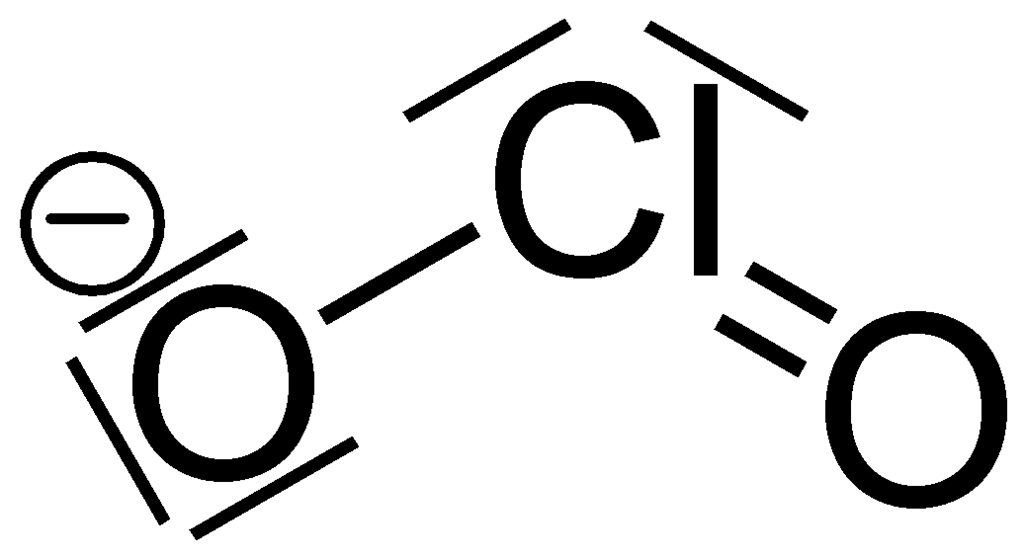
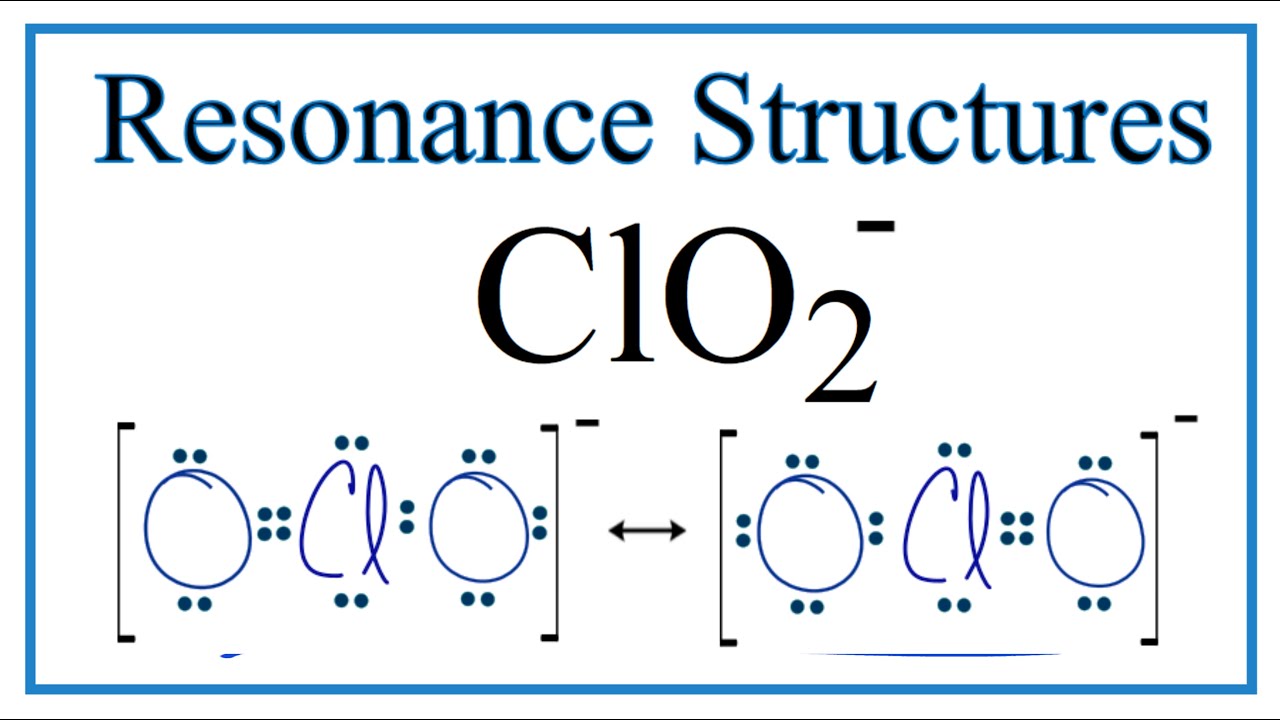
In the ClO 2- Lewis structure, there is one single bond and one double bond around the chlorine atom, with two oxygen atoms attached to it. The oxygen atom with a single bond has three lone pairs, and the oxygen atom with a double bond has two lone pairs. Also, there is a negative (-1) charge on the oxygen atom with a single bond. Contents Steps

Clo2 1 Lewis Structure
Hey Guys,We are back with one of the most requested videos, ClO2 Lewis structure. Chlorine Dioxide is made up of one chlorine atom and two oxygen atoms. To u.

Strutture di Lewis • Ione Clorito [ClO2] YouTube
The Lewis structure of ClO2 (chlorine dioxide) consists of a central chlorine atom bonded to two oxygen atoms. The chlorine atom has three lone pairs of electrons, while each oxygen atom has two lone pairs. The Lewis structure of ClO2 shows that it has a bent molecular geometry.
ClO2 Lewis Structure How to Draw the Lewis Structure for ClO2 YouTube
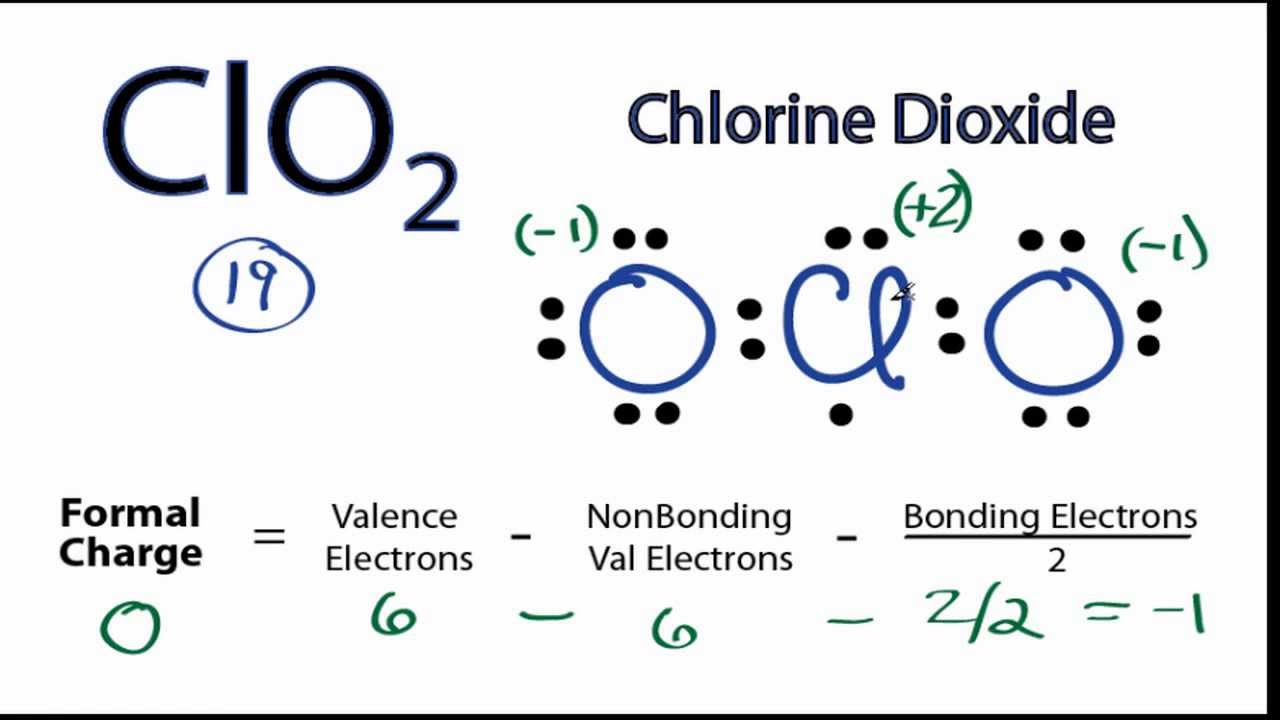
0:00 / 2:24 Lewis Structures, Introduction, Formal Charge, Molecular Geometry, Resonance, Polar or Nonpolar A step-by-step explanation of how to draw the ClO2 - Lewis Dot Structure.

How do we draw Lewis dot structure for ClO2 with proper explanation please Chemistry
The ClO2 Lewis structure has 19 valence electrons meaning that there will be an odd number of valence electrons in the structure. For the Lewis structure for ClO2 you should take formal charges into account to find the best Lewis structure for the molecule.

What is the bond order of ClO2?
This widget gets the Lewis structure of chemical compounds. Send feedback | Visit Wolfram|Alpha Get the free "Lewis Structure Finder" widget for your website, blog, Wordpress, Blogger, or iGoogle. Find more Chemistry widgets in Wolfram|Alpha.

Clo2 1 Lewis Structure
A quick explanation of the molecular geometry of ClO2 - (Chlorite ion) including a description of the ClO2 - bond angles.Looking at the ClO2- Lewis structure.

How can I draw the Lewis structure for ClO2?
ClO2- lewis structure comprises two oxygen (O) atoms and one chlorine (Cl) atom. The chlorine (Cl) atom is kept at the central position and the oxygen (O) atoms are in the surrounding position in the lewis diagram. The lewis dot structure of ClO2- contains 7 lone pairs and 3 bonded pairs.
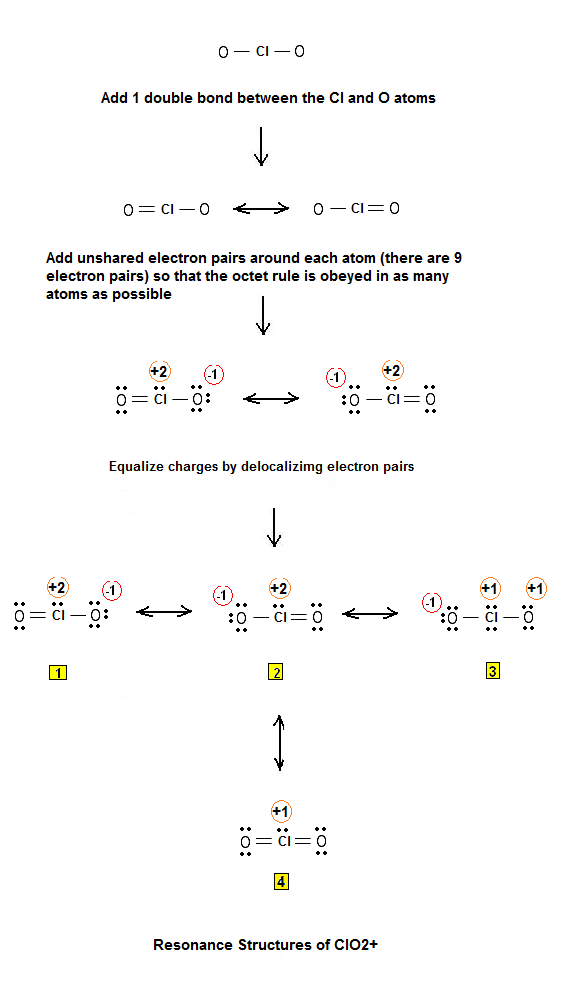
Lewis ElectronDot Structure for Chlorine Dioxide Ion (ClO2+) Chemistry Net
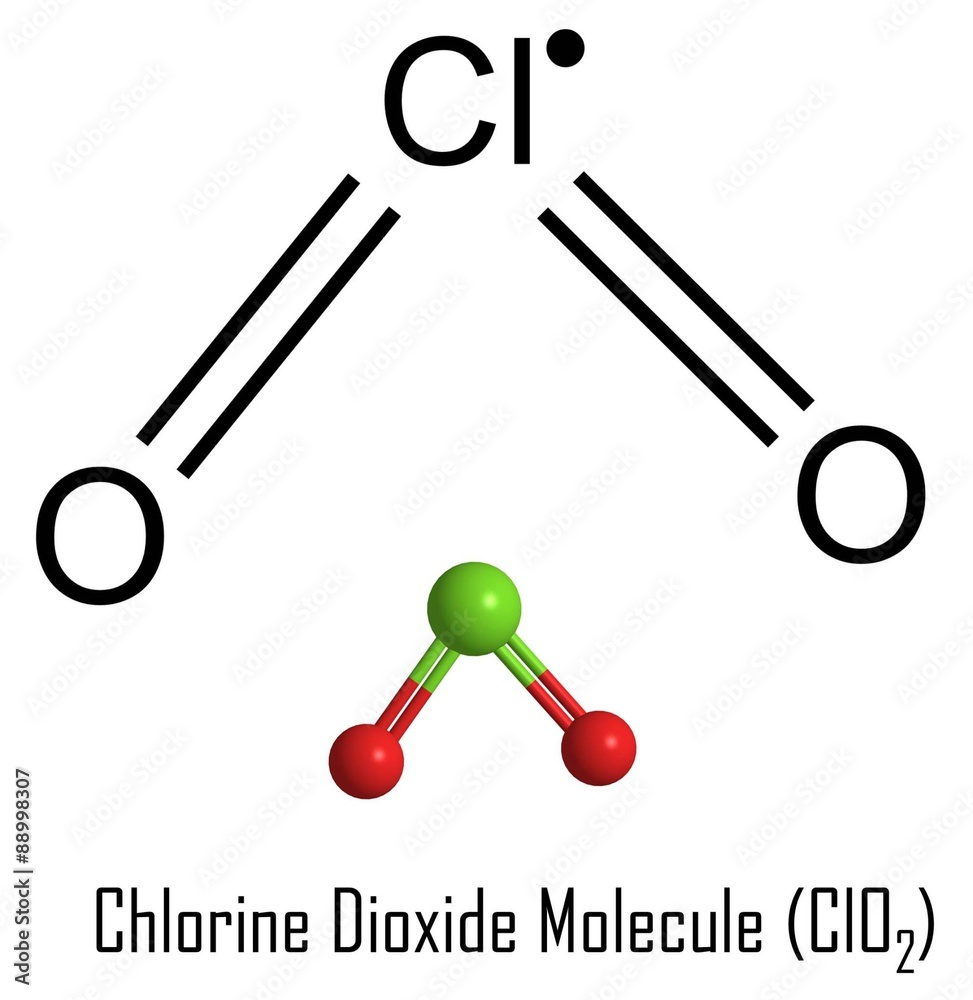
ClO2 lewis structure has a Chlorine atom (Cl) at the center which is surrounded by two Oxygen atoms (O). There are 2 double bonds between the Chlorine atom (Cl) and each Oxygen atom (O). There are 2 lone pairs on both the Oxygen atoms (O) and 1 lone pair & 1 unpaired electron on the Chlorine atom (Cl).

[Solved] Hybridisation of ClO2 9to5Science
Lewis structure of ClO2 (or Chlorine dioxide) contains two double bonds between the Chlorine (Cl) atom and Oxygen (O) atom. The Chlorine atom (Cl) is at the center and it is surrounded by 2 Oxygen atoms (O). The Chlorine atom has 1 lone pair and 1 unpaired electron, while both the Oxygen atoms have 2 lone pairs..

10.3 forbindelser af chlor Kemi Libretekster Anne Marie
Lewis Structure of Chlorine Dioxide (ClO2-) The Lewis structure is a pictorial representation of valence electrons taking part in the formation of bonds to produce a new molecule with new properties altogether. To begin drawing the Lewis structure of Chlorine dioxide, first, it is essential to draw one for the participating elements. For Chlorine,
Clo2 1 Lewis Structure
A step-by-step explanation of how to draw the ClO2 Lewis Dot Structure (Chlorine dioxide)The ClO2 Lewis structure has 19 valence electrons meaning that there.
What is the Lewis structure for resonance form of ClO_2^? Socratic
ClO 2- lewis structure has a Chlorine atom (Cl) at the center which is surrounded by two Oxygen atoms (O). There is 1 single bond and 1 double bond between the Chlorine atom (Cl) and each Oxygen atom (O). There are 2 lone pairs on double bonded Oxygen atom (O) and 3 lone pairs on single bonded Oxygen atom (O).

Clo2 1 Lewis Structure
In the Lewis structure for ClO2- we put Chlorine (Cl) at the center of the structure since it is the least electronegative. There are total of 20 valence electrons for the ClO2- Lewis structure. Remember that the negative sign counts as one valence electron.
Clo2 1 Lewis Structure
Lewis structure of ClO2- (or Chlorite ion) contains one double bond and one single bond between the Chlorine (Cl) atom and Oxygen (O) atom. The Chlorine atom (Cl) is at the center and it is surrounded by 2 Oxygen atoms (O). The Chlorine atom has 2 lone pairs, one Oxygen atom has 2 lone pairs and the other oxygen atom has 3 lone pairs.

ClO2 Lewis Structure, Geometry, Hybridization, and Polarity Techiescientist
The Lewis structure for ClO 2-is requires you to place Chlorine (Cl). Transcript: Let's do the ClO2- Lewis structure. Chlorine has 7 valence electrons. Oxygen has 6, we have two Oxygens; plus, we have a valence electron up here we need to add in for a total of 20 valence electrons. Chlorine's the least electronegative, it goes in the center.