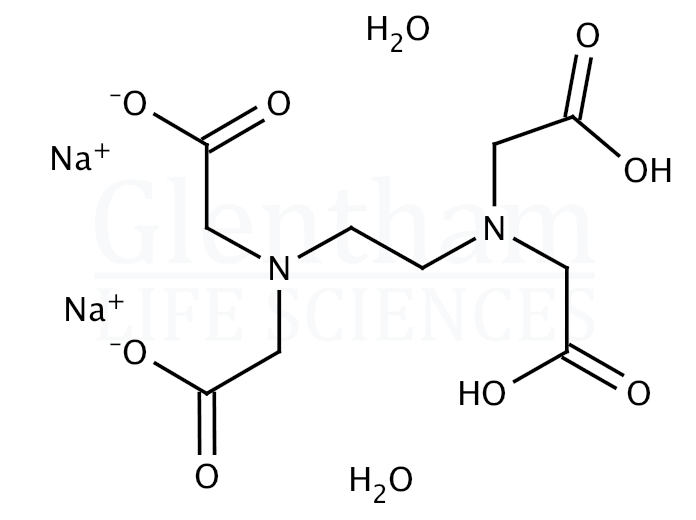
EDTA disodium salt dihydrate (CAS 6381926) Glentham Life Sciences
EDTA is commonly used as an efficient chelator of metal ion enzyme cofactors. It is highly soluble, optically inactive and does not interfere with most chemicals used in standard buffers making EDTA a common choice to generate metal-free conditions for biochemical and biophysical investigations. However, the controversy in the literature on metal-free enzyme activities achieved using EDTA or.

Complexes formation. The figure 2 shows the EDTA ligand binding to a... Download Scientific
Chelation Therapy Jeanne A. Drisko MD, in Integrative Medicine (Fourth Edition), 2018 EDTA EDTA is poorly absorbed from the GI tract (<5%) and, as a consequence, should only be administered by a parenteral route. 4 It is primarily distributed in extracellular fluids, which limit its capacity to chelate intracellular metals.
PPT EDTA Titrations Chapter 13 PowerPoint Presentation, free download ID6757871
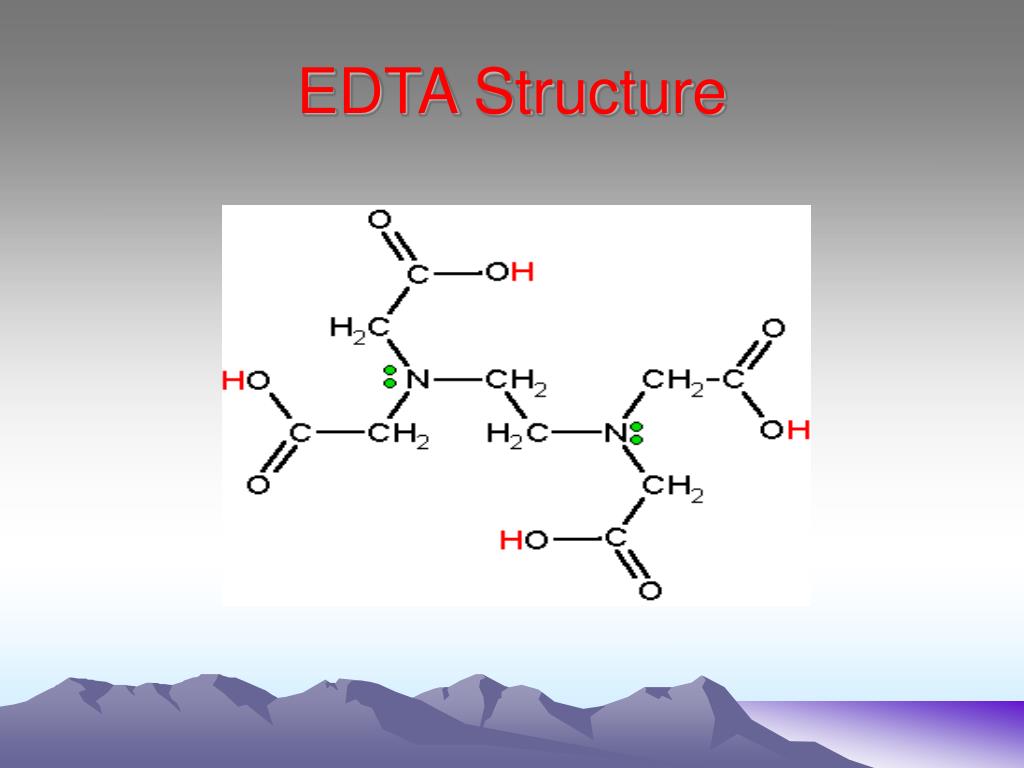
Ethylenediaminetetraacetic acid ( EDTA ), also called edetic acid after its own abbreviation, is an aminopolycarboxylic acid with the formula [CH 2 N (CH 2 CO 2 H) 2] 2. This white, water-insoluble solid is widely used to bind to iron (Fe 2+ /Fe 3+) and calcium ions (Ca 2+ ), forming water-soluble complexes even at neutral pH.

EDTA Full Form javatpoint
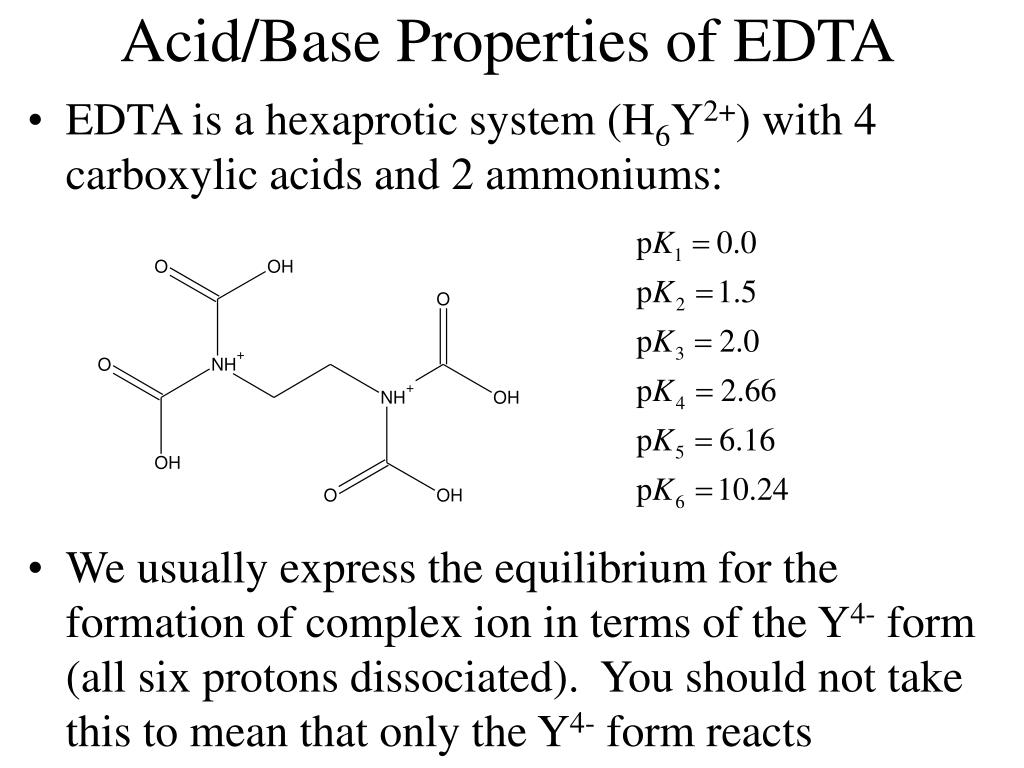
Ethylenediamine tetraacetic acid (EDTA) is a polyprotic acid containing four carboxylic acid groups and two amine groups with lone-pair electrons that chelate calcium and several other metal ions.

EDTA Full Form Ethylene Diamine TetraAcetic Acid StudyWoo
Ethylenediaminetetraacetic acid (EDTA) is a medication used in the management and treatment of heavy metal toxicity. It is in the chelating class of drugs. This activity outlines and reviews the indications, actions, and contraindications for EDTA as a valuable agent in managing lead toxicity.

EDTA Full Form, Structure and Form, Uses, Formula
Scope and Significance Ethylenediaminetetraacetic acid (EDTA) is a well known metal-chelating agent, extensively used for the treatment of patients who have been poisoned with heavy metal ions such as mercury and lead.

What is the full form of EDTA EDTA Full Form
The ADI for humans as set by JEFCA is 1.9 mg EDTA/kg bw (or 2.5 mg Ca-EDTA/kg bw), corresponding to 23.37 mg EDTA/kg bw for mice and is slightly higher than the one we used (21 mg/kg bw) 31.

619 EDTA Antikoagulantien US Blood Tissue (Biology)
This increased attention to EDTA resulted in studies of EDTA used as an antioxidant occurring in more recent years. The review contained three studies where EDTA was used as an antioxidant in order to treat psoriasis [19-20,22]. Another study in the review utilized EDTA as a chemical treatment in order to cause a decrease of beta lipoproteins .
EDTA South West London Pathology
TBE or Tris/Borate/EDTA, is a buffer solution containing a mixture of Tris base, boric acid and EDTA . In molecular biology, TBE and TAE buffers are often used in procedures involving nucleic acids, the most common being electrophoresis.
PPT EDTA Titrations PowerPoint Presentation ID234018
It is a popular chemical that goes by several names and is frequently utilized in medicinal and industrial applications. Ferdinand Munz was the first to synthesize this chemical in 1935. It is a colorless, crystalline, slightly soluble organic molecule utilized in biology and inorganic chemistry. It is a chelating agent.

PPT EDTA Titrations Chapter 13 PowerPoint Presentation, free download ID6757871
TAE buffer TAE buffer is a buffer solution containing a mixture of Tris base, acetic acid and EDTA. In molecular biology, it is used in agarose electrophoresis typically for the separation of nucleic acids such as DNA and RNA. [1] It is made up of Tris-acetate buffer, usually at pH 8.3, and EDTA, which sequesters divalent cations.

The molecule structure of calcium chelated with eDTa and Pa.... Download Scientific Diagram
TE buffer is a commonly used buffer solution in molecular biology, especially in procedures involving DNA, cDNA or RNA. "TE" is derived from its components: Tris, a common pH buffer, and EDTA, a molecule that chelates cations like Mg 2+. The purpose of TE buffer is to solubilize DNA or RNA, while protecting it from degradation. Recipe

Sigma Aldrich EDTA FOR MOLECULAR BIOLOGY Cas 6381926 Sigma Aldrich
Applications- EDTA : EDTA is a popular chelating agent for divalent ions, that is widely used in biochemistry, molecular biology and cell biology. EDTA is an abbreviation for EthyleneDiamineTetraAcetic ac (and many other related molecules). EDTA is an amino acid widely used to sequester di- and trivalent metal ions (Ca2+ and Mg2+ for example).

What is the Full Form of EDTA? I Leverage Edu
1. Introduction. Divalent metal ions including Mg 2+, Cu 2+, Fe 2+, Mn 2+, Ni 2+, and Zn 2+ play prominent roles in enzymatic catalysis. The small organic compound ethylene diamine tetraacetic acid or commonly known as EDTA is frequently used to chelate these metal ions to investigate enzyme function in the absence of metal co-factors.

What is the full form of EDTA? EDTA full form
Bulk and Prepack available | Ethylenediaminetetraacetic acid disodium salt dihydrate for electrophoresis, for molecular biology |EC Number: 205-358-3; Synonym: Disodium ethylenediaminetetraacetate dihydrate, EDTA-Na2; Linear Formula: C10H14N2Na2O8 · 2H2O | Explore related products, MSDS, application guides, procedures and protocols at Sigma Aldrich - a one stop solution for all your research.

Disodium Edetate Disodium EDTA Drug Molecule. Skeletal Formula. Stock Vector Illustration of
Ethylenediaminetetraacetic acid is a molecule that we know as a chelating agent. Furthermore, it is something that has a claw-like structure that we use to stick and grab other molecules. On one hand, its some part sticks to calcium and on the other hand, some of it sticks to metals. Moreover, doctors prescribe them to clear toxins from the body.