
How Many Valence Electrons Does Neon(Ne) Have?
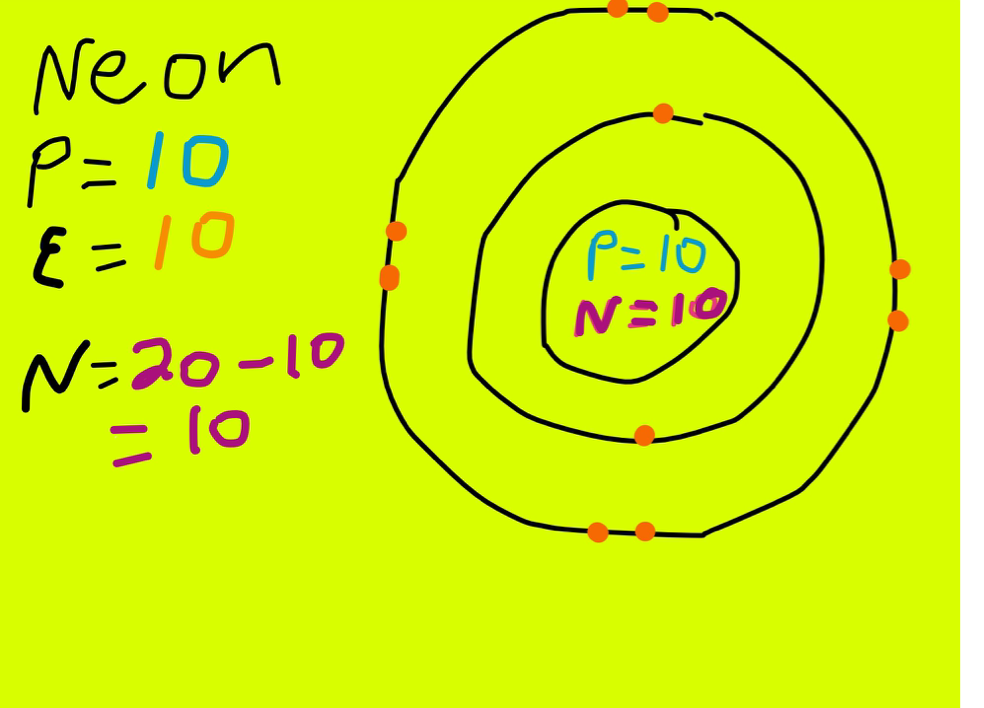
Therefore, neon has 8 valence electrons. Answer b Calcium has electrons in the first, second, third, and fourth energy levels, as indicated by the leading red 1, 2 's, 3 's, and 4, respectively. Valence electrons are those found in the highest occupied energy level.

What Are Valence Electrons? Definition and Periodic Table 8th Grade
Step 1) Figure out how many electrons the molecule must have. Carbon has 4 valence electrons. Each oxygen has 6 valence electrons. The -2 charge means that there are 2 extra electrons. Total: 4 + (3 × 6) + 2 = 24 electrons. The final answer MUST have this number of electrons‼!
PPT Unit 3 PowerPoint Presentation, free download ID5685070
1). You can effortlessly find every single detail about the elements from this single Interactive Periodic table. 2). You will get the detailed information about the periodic table which will convert a newbie into pro. 3). You will also get the HD images of the Periodic table (for FREE).

How to find the Valence Electrons for Neon (Ne) YouTube
Get ready to find out how many valence electrons neon has! In this video, we'll explore the fascinating world of chemistry and discover why valence electrons.

Neon Electron Configuration YouTube
The arrangement of electrons in neon in specific rules in different orbits and orbitals is called the electron configuration of neon. The electron configuration of neon is [ He] 2s 2 2p 6, if the electron arrangement is through orbitals. Electron configuration can be done in two ways. Electron configuration through orbit (Bohr principle)
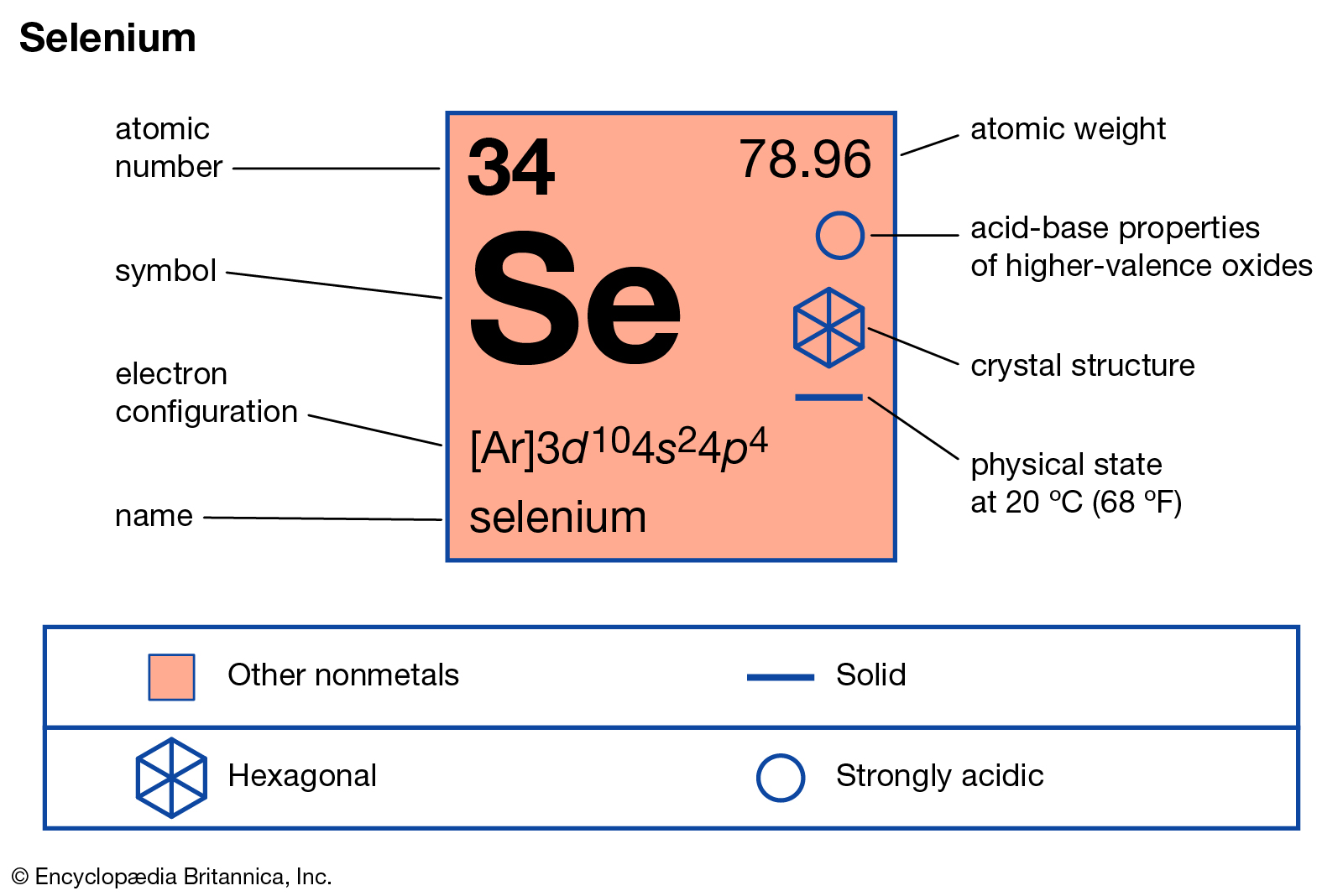
Valency of Selenium Archives Dynamic Periodic Table of Elements and
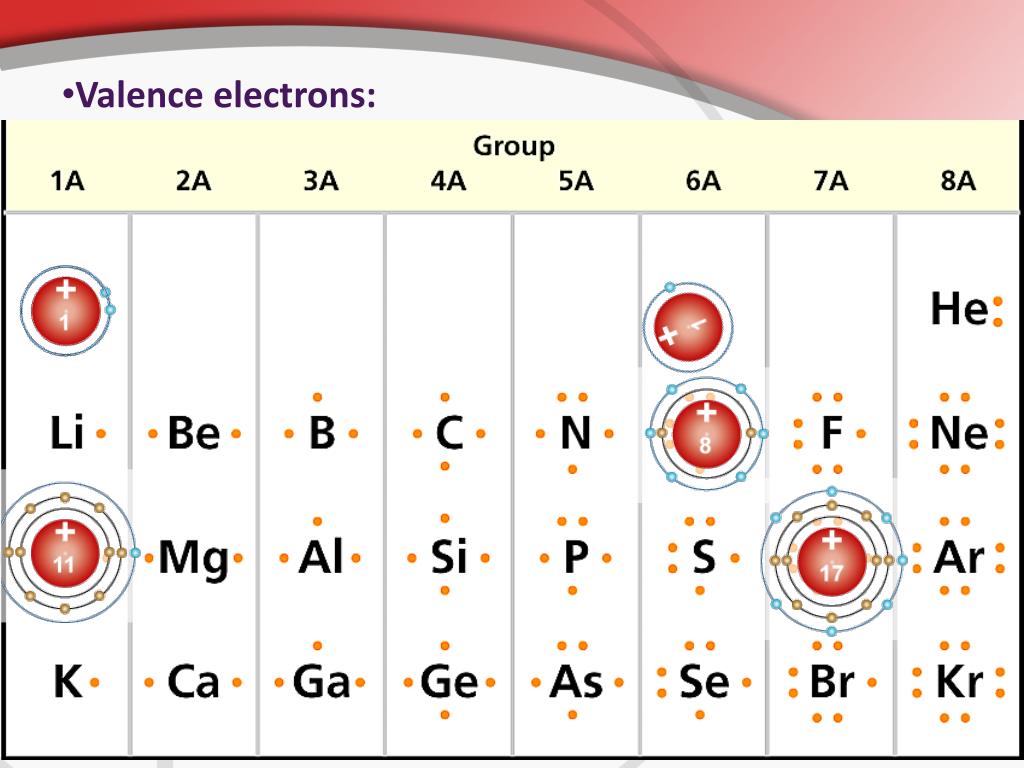
Each element has a number of valence electrons equal to its group number on the Periodic Table. Figure %: The periodicity of valence electrons This table. Helium (He) and Neon (Ne) have outer valence shells that are completely filled, so neither has a tendency to gain or lose electrons. Therefore, Helium and Neon, two of the so-called Noble.
How Many Valence Electrons Are in the Neon Family
Manahil Ahmed 9 years ago If helium is in group 8 why does it not have 8 valence electrons . And when two hydrogen atoms combine they make a molecule and then have a total of 2 valence electrons if they are then stable then why are the elements in group 2 not stable with 2 valence electrons ? • Comment ( 13 votes) Upvote Downvote Flag Just Keith
How many valence electrons does sulfur(S) have?
Explanation: Neon, Z = 10, has eight valence electrons. This closed shell configuration makes neon supremely difficult to oxidize, and difficult to reduce. The inertness, the lack of reactivity, of this Noble Gas, is a function of its electronic configuration. Answer link To which group of the Periodic Table does neon belong?
silvercraftdesignstudios How Many Valence Electrons Does Antimony Have
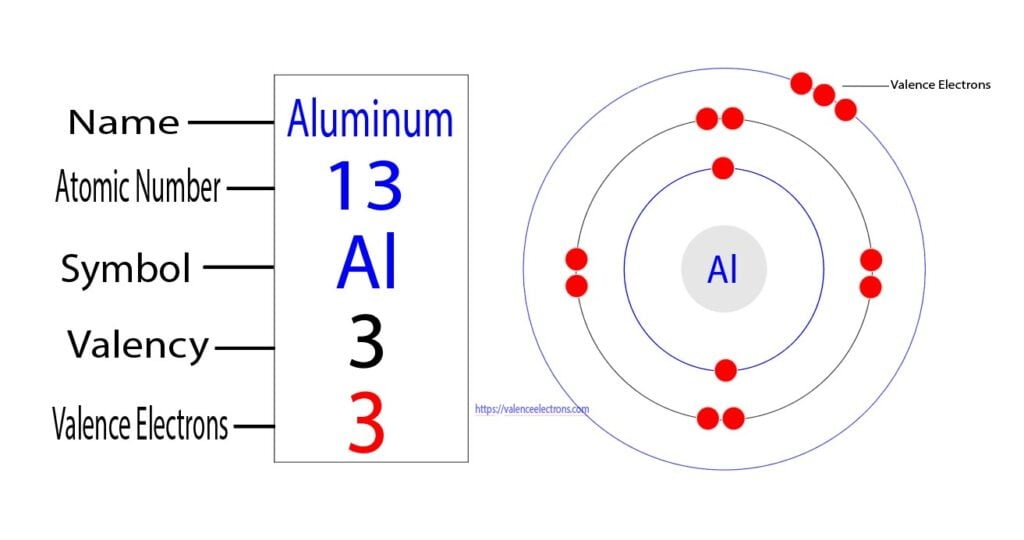
Valence electrons are the electrons present in the outermost shell of an atom. You can easily determine the number of valence electrons an atom can have by looking at its Group in the periodic table. For example, atoms in Groups 1 and 2 have 1 and 2 valence electrons, respectively. Atoms in Groups 13 and 18 have 3 and 8 valence electrons.
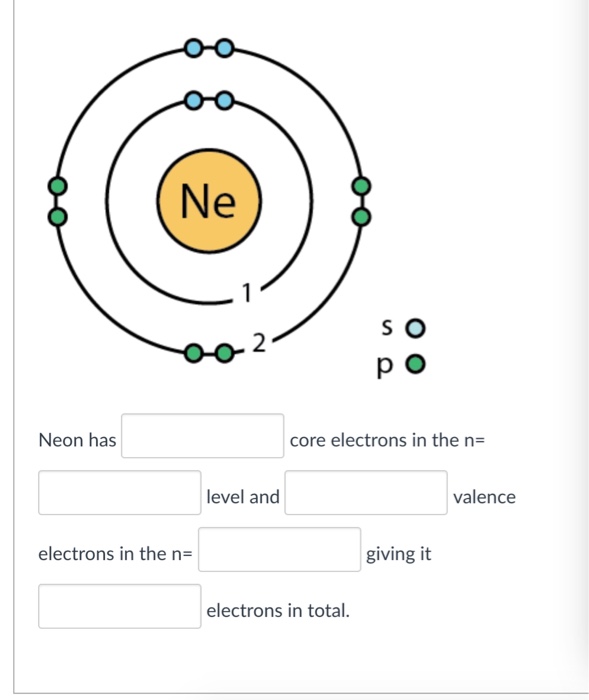
Solved Ne 2 p o Neon has core electrons in the n level and
Please enable JavaScript to access the full features of the site. Glossary Allotropes Some elements exist in several different structural forms, called allotropes. Each allotrope has different physical properties. For more information on the Visual Elements image see the Uses and properties section below. Move to Fluorine Move to Sodium > Neon
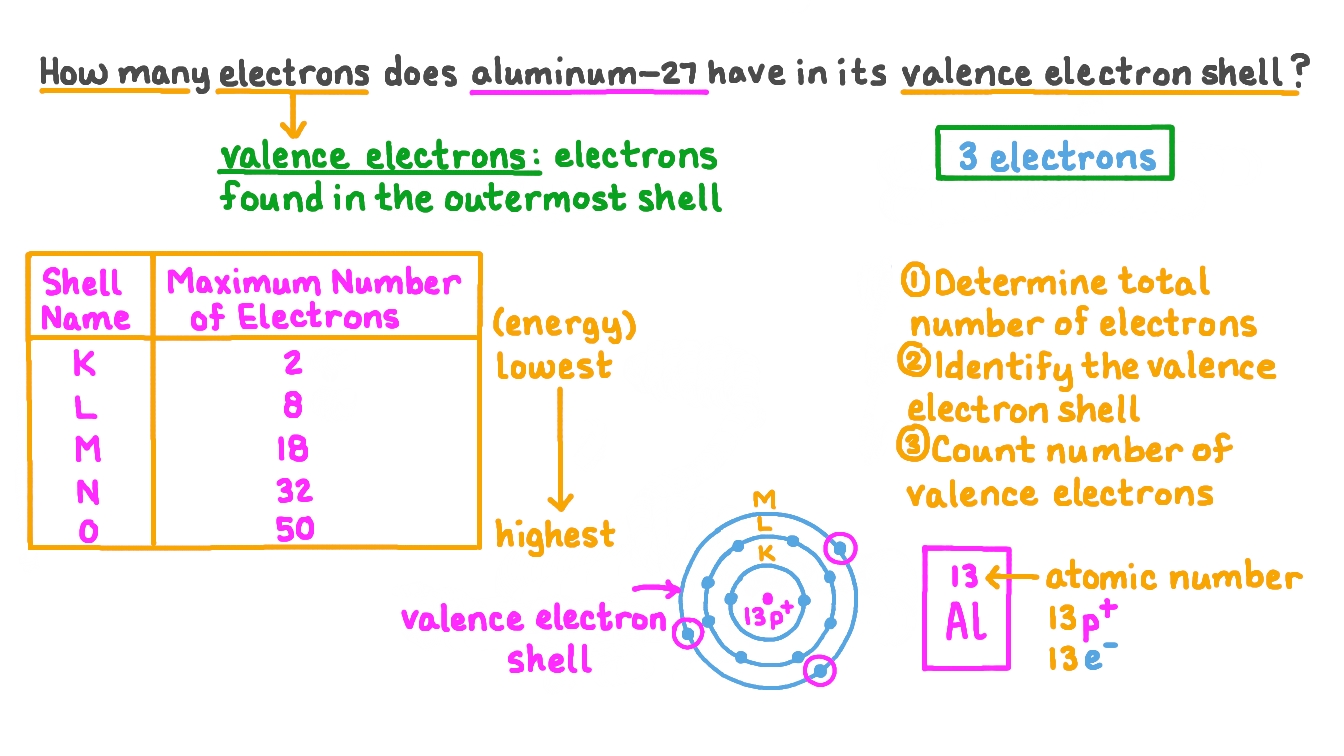
Question Video Determining the Number of Electrons in the Valence
Here is a table of element valences. Remember that an element's electron cloud will become more stable by filling, emptying, or half-filling the shell. Also, shells don't stack neatly one on top of another, so don't always assume an element's valence is determined by the number of electrons in its outer shell. Table of Element Valences Sources
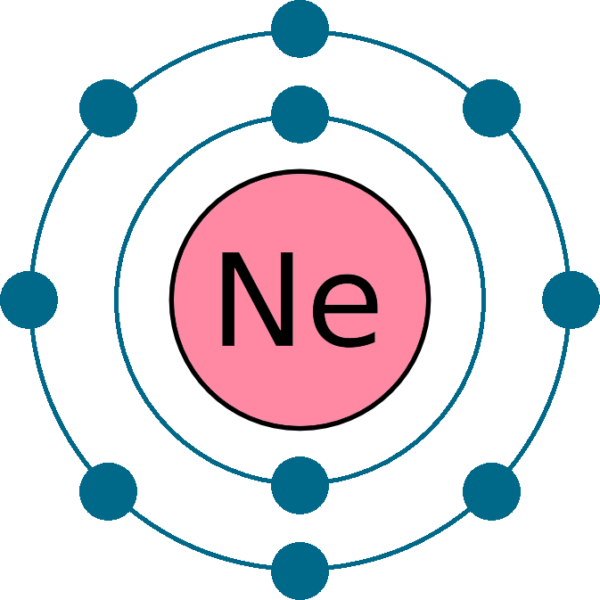
Electron Configuration Of Neon My XXX Hot Girl
The Bohr model shows the atom as a central nucleus containing protons and neutrons, with the electrons in circular electron shells at specific distances from the nucleus, similar to planets orbiting around the sun.
How Many Valence Electrons Does Helium (He) Have? [Valency of He]
There are four simple steps to find out the valence electrons for neon atom which are: Step 1: Find the Atomic Number To find out the atomic number of neon, we can use the periodic table. With the help of the periodic table, we can easily see that the atomic number of neon is 10.

Neon Protons Neutrons Electrons Electron Configuration
About Transcript Valence electrons are the electrons in the outermost shell, or energy level, of an atom. For example, oxygen has six valence electrons, two in the 2s subshell and four in the 2p subshell. We can write the configuration of oxygen's valence electrons as 2s²2p⁴. Created by Sal Khan. Questions Tips & Thanks
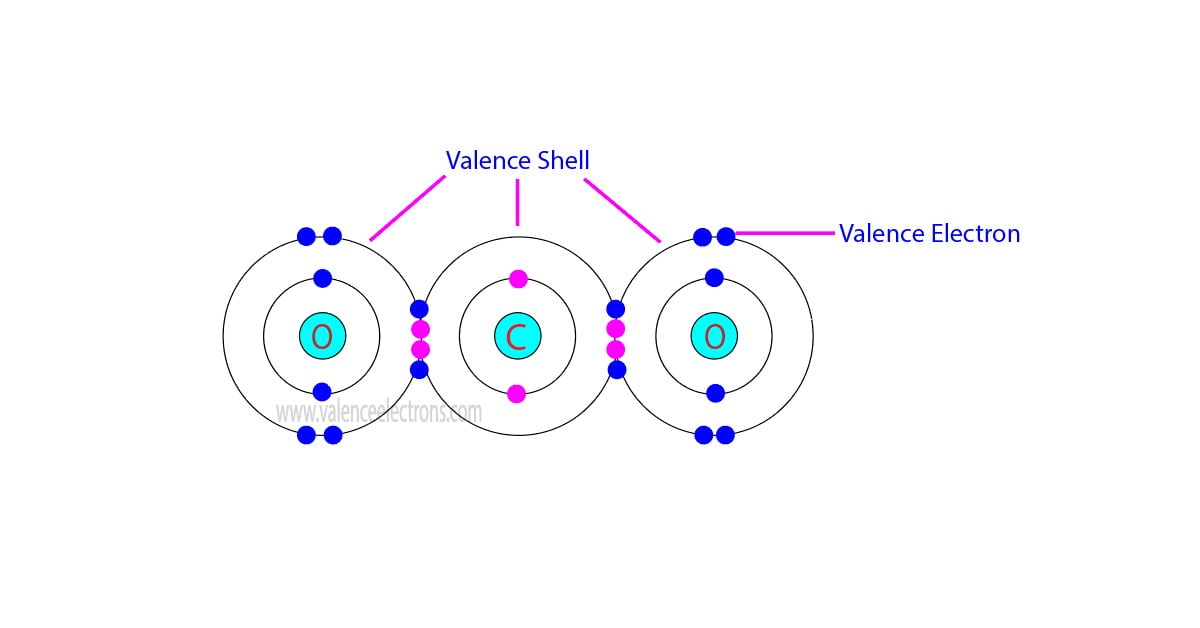
How many valence electrons does neon(Ne) have?
It simply depicts the theory that the first two orbital the 1s and the 2s are having 2 electrons respectively. The remaining 6 electrons have been held by the 2p orbital. How Many Valence Electrons Does Neon Have? Neon basically has the 8 valence electrons as its full octet and this is what makes neon stable in the terms of a noble gas.

How many valence electrons does neon(Ne) have?
There are two ways to find the number of valence electrons in Neon (Ne). The first is to use the Periodic Table to figure out how many electrons Neon has in its valence shell. To do.